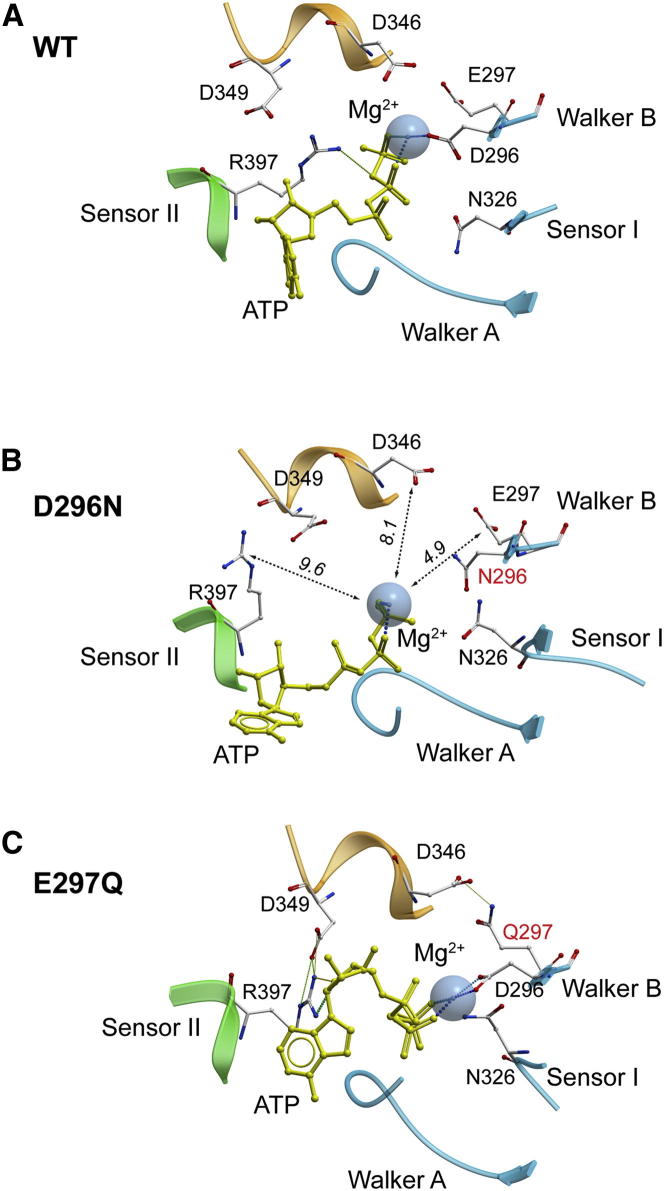
Figure 5.
Dilatation of Catalytic Pockets in the Walker B Mutants in mkTIP49 Hexamers
The 30 ns conformation of the wild-type protein (A) is aligned with that of the D296N (B) and E297Q (C) mutant proteins. The GDLLDR motif from the adjacent protomer is colored in orange. See also Figure S4 for details of D296, E297, D346, and R397 dynamics. Note that during MD simulations the phosphate chain remains the most stably bound part of ATP within the catalytic pockets of mkTIP49. The nucleoside moiety of ATP, however, can also undergo significant thermal fluctuations (compare A, B, and C). This could be attributed to the smaller number of H-bonds that are formed with the nucleoside part of ATP relative to the phosphate chain, which is remarkably well coordinated by Walker A, Sensor II, and Mg2+ (see also Figure 2).