Alkaptonuria (AKU) is an ultra-rare disease developed from the lack of homogentisate 1,2-dioxygenase activity, causing an accumulation of homogentisic acid (HGA) in pigmented deposits (ochronosis) in connective tissues, especially in the joints and heart. AKU is mostly asymptomatic in early life, with arthropathy and cardiovascular symptoms appearing in later decades of life. Cardiovascular involvement has been described in as many as 40% of AKU patients (mean and median age of detection being 54 and 52 years, respectively) [1]. Alkaptonuric ochronosis can be treated symptomatically during the early stages, whereas for end stages total joint and heart valve replacements may be required [1–3], but no specific cure exists at the moment, although a phase II clinical trial with nitisinone is in progress.
Thanks to the use of our in vitro, cell and tissue AKU models [4–10], we recently provided experimental evidence that AKU is a secondary serum amyloid A (SAA)-based amyloidosis [8]. A co-localization of ochronotic pigment and SAA-amyloid was also reported [8].
In the present work, using Congo Red (CR) birefringence and immunofluorescence, we report for the first time the presence of SAA-amyloidosis in the stenotic aortic valve of an AKU patient. Light microscopy revealed a co-localization of ochronotic pigment and SAA-amyloid, tissue calcification, lipid oxidation, inflammation, and tissue degeneration.
Alkaptonuric specimen was obtained from a patient (Table 1) who underwent biologic aortic valve replacement. AKU patient had been previously diagnosed for secondary amyloidosis as SAA-amyloid had been detected in her cartilage and synovia [8]. Echocardiogram revealed severe aortic stenosis with an aortic transvalvular mean gradient of 53 mm Hg, a peak transvalvular gradient of 89, a peak of systolic velocity (m/s) of 4.7 and valve area of 0.82 cm2 and an AVA indexed for body weight of 0.52 cm2. Inter-ventricular septal thickness was 12 mm. Left ventricular systolic function was preserved. In view of the severe aortic stenosis the patient underwent elective aortic valve replacement. The postoperative course was uneventful and the patient recovered well from surgery at one-year follow-up.
Table 1.
Clinical history of the patient.
| Sex year of birth | Female 1948 | |||
|---|---|---|---|---|
| 1985 | Meniscus break (L) | |||
| 1986 | 3 lumbosciatica attacks | Reduction in spinal discs | Spondylolisthesis | AKU diagnosis |
| 1987 | Low proteins diet: no results | Tiroxina with C protein | ||
| 1989 | Meniscus break (R) | |||
| 1994 | Achilles' tendon thickening (L) | |||
| 1997 | Drooling | Hiatus hernia | Esophagitis (II degree) | |
| 1998 | Ulna and radial head capitellum break (R) | |||
| 2001 | Knee rotuleus tendon break (L) | |||
| 2003 | Macroglossia | |||
| 2005 | Thyroid removal | Hip prosthesis (R) with bone replacement | ||
| 2006 | Hip prosthesis (L) | |||
| 2008 | Tachycardia | Aortic valve stenosis | ||
| 2010 | 2 ribs break | |||
| 2011 | Aortic valve replacement | |||
| AKU features | Elbows and heels bursitis | Shoulder pain | 15 cm decline of height | Amyloid deposits in cartilage, synovia and aortic valve |
| SAA plasma level | 110 mg/L | |||
| SAP plasma level | 50 μg/L | |||
| HGA plasma level | 11 μg/mL | |||
| HGA urine excretion | 8.6 g/day | |||
| HGD Mutation | G270R |
After surgery AKU aortic valve sections were stained with eosin/hematoxylin, CR stained for amyloid detection or used for immunofluorescence staining with anti-SAA and anti-4-hydroxy-2-nonenal (4-HNE) antibodies.
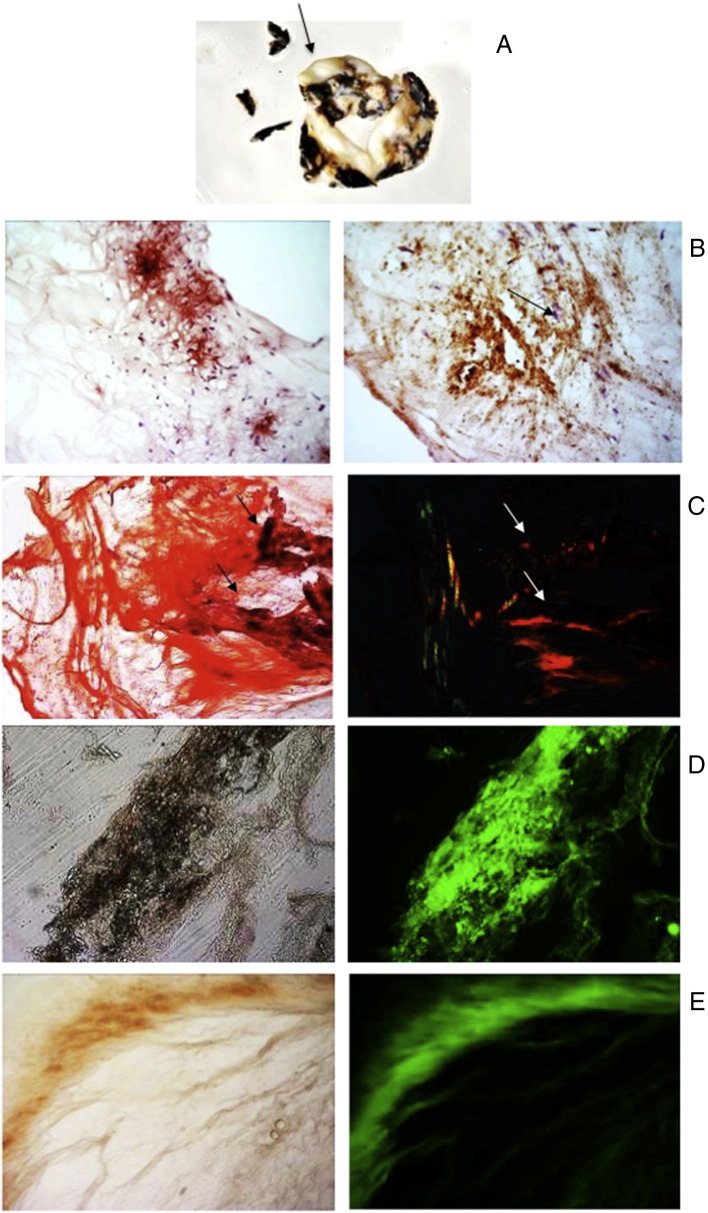
At a glance, the aortic valve revealed massive deposits of ochronotic pigment with calcification in the cusps (Fig. 1A). Sclerotic change in the cusps, and shrinkage of the non-coronary cusp, impeding normal coaptation of the aortic valve, associated with diffuse ochronotic deposition, appeared to be the causative lesions of the need for aortic valve replacement. Ochronosis was associated with areas of valvular calcification. Light microscopy examinations consistently showed severe calcification involving surface endothelium (Fig. 1B). CR staining detected the presence of diffuse amyloid, mainly located in densely sclerotic and poorly vascularised scar tissue and co-localization of amyloid and ochronotic pigment was visible in close proximity to calcific deposits (Fig. 1C). Co-localization of SAA deposition with ochronotic pigmentation was detected (Fig. 1D). Major products of lipid peroxidation (LPO) were detected in AKU valve sections incubated with anti-4-HNE antibody (Fig. 1E). LPO were perfectly superimposing to pigmented areas, suggesting the association of intraleaflet ochronosis and oxidative stress (Fig. 1E). AKU valve also contained macrophages in the subendothelial layer of the fibrosa, in the vicinity of ochronotic deposits, and along the lamina elastic (Fig. 1B). An evident co-localization between oxidized lipids, inflammation and ochronosis suggested that LPO might play a role in the disease process.
Fig. 1.
A) Macroscopic aspect of AKU aortic valve showing a rough surface of the leaflets and ochronotic pigmentation on the surface. A thickening of the central areas of the cusps with nodule-like structures is visible (arrow). B) Light microscopy (hematoxylin and eosin staining) examinations of AKU aortic valve with intense inflammatory cell infiltrate that includes large cells macroscopically corresponding to macrophages (arrows). Magnification 20 ×. C) Congo Red staining of AKU aortic valve amyloid deposits. Left: unpolarized light. Valve tissue was ever stained red (“congophilic”); Right: polarized light. The pigmented areas (arrows) were also birefringent, indicating overlapping of ochronosis and amyloid. Magnification 40 ×. D) SAA was present in AKU aortic valve amyloid deposits. Positive staining for SAA-amyloid was particularly intense in correspondence of ochronotic pigmentation. Magnification 20 ×. E) Immunoreactivity for 4-HNE in AKU aortic valve leaflet. The presence of 4-HNE in the AKU valve was uniformly diffused and the distribution of 4-HNE-positive area was perfectly superimposing to ochronotic pigmented areas. The presence of lipid peroxidation and ochronotic pigment was found to be strictly related to areas of lymphocytes accumulation. Magnification 20 ×.
Our present investigation confirmed the hitherto unsuspected existence of AKU-linked secondary amyloidosis, involving also the cardiac district. The present case report is therefore a new rare case of SAA amyloidosis in the heart.
The striking co-localization of pigment and amyloid suggested that HGA might be involved in amyloid deposition in the heart.
In conclusion, the emerging view in this AKU case is that valve calcification represents a meeting of lipid oxidation with chronic inflammation and amyloid deposition. In AKU cases, surgical replacement of aortic valves results in significant improvement. Physicians and surgeons should be aware of multiple system involvement in this disorder, as early recognition and appropriate treatment may significantly improve the quality of life in AKU patients.
Acknowledgments
This work has been supported by TELETHON ITALY grant GGP10058.
The authors thank the alkaptonuric patient who generously donated her aortic valve for the present study, AimAKU (Associazione Italiana Malati di Alcaptonuria, ORPHA263402), Toscana Life Sciences Orphan_1 project and Fondazione Monte dei Paschi di Siena 2008–2010.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Thakur S., Markman P., Cullen H. Choice of valve prosthesis in a rare clinical condition: aortic stenosis due to alkaptonuria. Heart Lung Circ. 2013;22(10):870–872. doi: 10.1016/j.hlc.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Hiroyoshi J., Saito A., Panthee N. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95:1076–1079. doi: 10.1016/j.athoracsur.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Lok Z.S., Goldstein J., Smith J.A. Alkaptonuria-associated aortic stenosis. J Card Surg. 2013;28:417–420. doi: 10.1111/jocs.12129. [DOI] [PubMed] [Google Scholar]
- 4.Braconi D., Bernardini G., Bianchini C. A biochemical and proteomic characterization of alkaptonuric chondrocytes. J Cell Physiol. 2012;227:3333–3343. doi: 10.1002/jcp.24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braconi D., Bianchini C., Bernardini G. Redox-proteomics of the effects of homogentisic acid in an in vitro human serum model of alkaptonuric ochronosis. J Inherit Metab Dis. 2011;34:1163–1176. doi: 10.1007/s10545-011-9377-6. [DOI] [PubMed] [Google Scholar]
- 6.Braconi D., Laschi M., Amato L. Evaluation of anti-oxidant treatments in an in vitro model of alkaptonuric ochronosis. Rheumatology (Oxford) 2010;49:1975–1983. doi: 10.1093/rheumatology/keq175. [DOI] [PubMed] [Google Scholar]
- 7.Braconi D., Laschi M., Taylor A.M. Proteomic and redox-proteomic evaluation of homogentisic acid and ascorbic acid effects on human articular chondrocytes. J Cell Biochem. 2010;111:922–932. doi: 10.1002/jcb.22780. [DOI] [PubMed] [Google Scholar]
- 8.Millucci L., Spreafico A., Tinti L. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim Biophys Acta. 1822;2012:1682–1689. doi: 10.1016/j.bbadis.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinti L., Spreafico A., Braconi D. Evaluation of antioxidant drugs for the treatment of ochronotic alkaptonuria in an in vitro human cell model. J Cell Physiol. 2010;225:84–91. doi: 10.1002/jcp.22199. [DOI] [PubMed] [Google Scholar]
- 10.Tinti L., Taylor A.M., Santucci A. Development of an in vitro model to investigate joint ochronosis in alkaptonuria. Rheumatology (Oxford) 2011;50:271–277. doi: 10.1093/rheumatology/keq246. [DOI] [PubMed] [Google Scholar]