Table 1.
Optimization for cross-coupling with n-pentyl Grignard.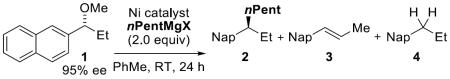
| Entry | Ni catalyst (mol %) | X | Yield 2 [%][a] | ee 2 [%][b] | es 2 [%][c] | Yield 3 [%][a] | Yield 4 [%][a] |
|---|---|---|---|---|---|---|---|
| 1 | no Ni, no ligand | I | <5[d] | – | – | <5[d] | <5[d] |
| 2 | Ni(acac)2 (10), no ligand | I | <5[d] | – | – | <5[d] | <5[d] |
| 3 | Ni(acac)2 (10), rac-BINAP (10) | I | <5[d] | – | – | <5[d] | <5[d] |
| 4 | Ni(acac)2(10), DPEphos (10) | I | 28 | 86 | 91 | 27 | 12 |
| 5 | Ni(acac)2 (10), PPh3 (10) | I | 8 | 82 | 86 | 55 | 14 |
| 6 | Ni(acac)2 (10), dppp (10) | I | 31 | 45 | 47 | 12 | 14 |
| 7 | Ni(acac)2 (10), dppe (10) | I | 69 | 66 | 69 | <5 | <5 |
| 8 | Ni(acac)2 (10), dppe (15) | I | 95 | 61 | 64 | <5 | <5 |
| 9 | Ni(acac)2 (10), dppe (20) | I | 0–90[e] | 83[f] | 87[f] | <5 | <5 |
| 10 | Ni(acac)2 (10), dppe (22) | I | <5[d] | – | – | <5[d] | <5[d] |
| 11 | Ni(cod)2 (10), dppe (10) | I | <5[d] | – | – | <5[d] | <5[d] |
| 12 | Ni(dppe)CI2 (10) | I | 89 | 55 | 58 | <5 | <5 |
| 13 | Ni(dppe)CI2 (10) | CI | 96 | 51 | 54 | 5 | <5 |
| 14 | Ni(dppe)CI2 (10) | Br | 95 | 91 | 96 | <5 | <5 |
| 15 | Ni(dppe)CI2 (5) | Br | 85 | 93 | 98 | <5 | <5 |
| 16 | Ni(dppe)CI2 (2) | Br | 97[g] | 96 | >99 | <5 | <5 |
Determined by 1H NMR analysis using internal standard (PhSiMe3).
Determined by chiral SFC.
[c] Enantiospecificity (es) = eeproduct/eestarting material × 100%.
Recovered unreacted 1.
Reaction was irreproducible: run 1: <5% yield; run 2: <5% yield; run 3: 90% yield, 85% ee, 89% es; run 4: <5% yield; run 5: <5% yield; run 6: 84% yield, 68% ee, 72% es; run 7: 90% yield, 94% ee, 99% es;
Average of runs 3, 6, and 7.
Isolated yield.
