Table 2.
Scope of cross-coupling reaction of alkylmagnesium bromides.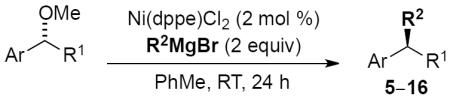
| Entry | Product | Yield [%][a] | S.M. ee [%][b] | Prod ee [%][b] | es [%] | ||
|---|---|---|---|---|---|---|---|
| 1 |
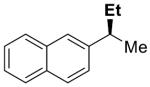
|
5 | 80 | 92 | 91 | 99 | |
| 2 | 6 | R2 = nPr | 93 | 97 | 97 | >99 | |
| 3 | 7 | R2 = nPent | 91 | 97 | 97 | >99 | |
| 4 |
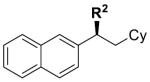
|
8 | R2 = (CH2)3Ph | 88 | 97 | 97 | >99 |
| 5 [c] | 9 | R2 = 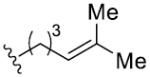
|
81 | 97 | 97 | >99 | |
| 6[d] | 10 | R2 = (CH2)3CF3 | 68 | >99 | 97 | 97 | |
| 7 | 11 | R2 = iBu | 40 | 97 | 90 | 93 | |
| 8 |
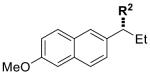
|
12 | R2 = nPr | 80 | 93 | 92 | 99 |
| 9 | 13 | R2 = (CH2)3Ph | 88 | 93 | 93 | >99 | |
| 10[c] |
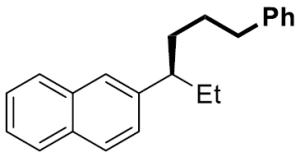
|
14 | 93 | 97 | 97 | >99 | |
| 11[e] |
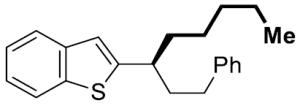
|
15 | 54 | 99 | 96 | 97 | |
| 12[f] |
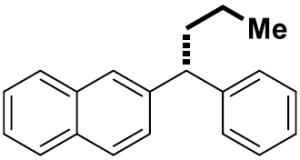
|
16 | 67 | >99 | 91 | 91 | |
Calculated yield after silica gel chromatography.
Determined by chiral SFC chromatography, S.M. = starting material, Prod. = product.
5 mol % Ni(dppe)CI2.
10 mol % Ni(dppe)CI2.
Ni(dppe)CI2 was added in two aliquots of 5 mol %; see SI for details.
5 °C; 48 h; 15% 2-benzylnapthalene byproduct.
