Table 3.
Scope of cross-coupling reaction of arylmagnesium bromides.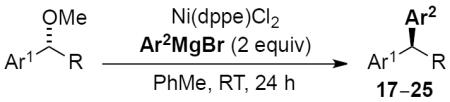
| Entry | Product | Ni(dppe)CI2 [mol %] | Yield [%][a] | S.M. ee [%][b] | Prod ee [%][b] | es [%] | ||
|---|---|---|---|---|---|---|---|---|
| 1 |
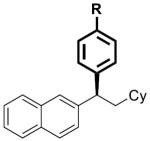
|
17 | R = H | 2 | 67 | >99 | 91 | 92 |
| 2 | 18 | R = NMe2 | 5 | 80 | >99 | nd[c] | nd[c] | |
| 3 | 19 | R = OMe | 2 | 86[d] | >99 | 97 | 97 | |
| 4 | 20 | R = F | 2 | 82[d] | >99 | 87 | 88 | |
| 5 |
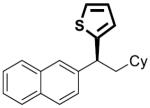
|
21 | 10 | 76 | >99 | 93 | 94 | |
| 6 |
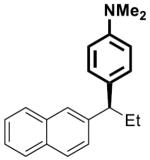
|
22 | 5 | 80 | >99 | 85 | 86 | |
| 7 |
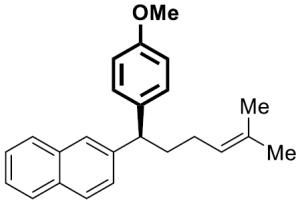
|
23 | 4 | 92[d] | 96 | 88 | 92 | |
| 8 |
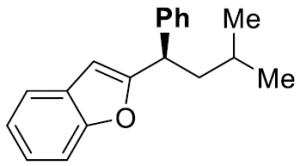
|
24 | 10 | 71 | 97 | 95 | 98 | |
| 9[e] |
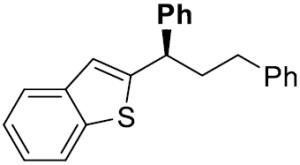
|
25 | 20 | 76 | 99 | 93 | 94 | |
