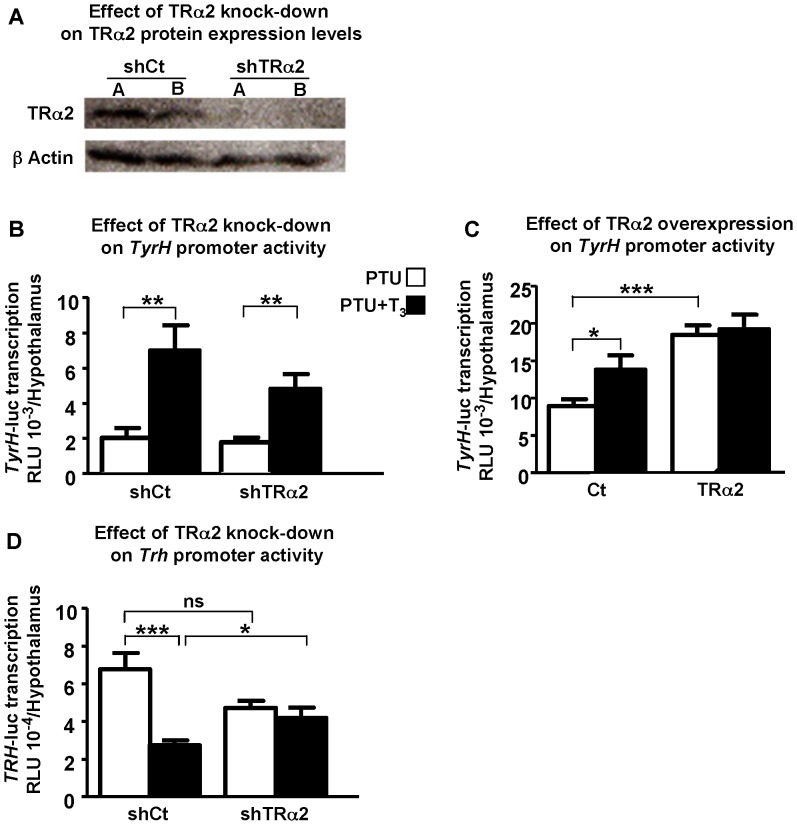
Figure 2. Transcriptional effect of TRα2 knockdown on positively and negatively regulated T3 target genes in vivo.
A: Knockdown of TRα2. TRα2 expression levels were analyzed on hypothalami (48 h after transfection) of hypothyroid 2-day old mice by western-blot using anti-TRα2 antibody. A decrease in TRα2 protein expression level is observed with shTRα2 compared to control (shCt). Pups were transfected in the hypothalami with 400 ng/pup of shCt (empty pCMV-H1 vector (shCt)) or shTRα2 (mixture of 200 ng pCMV-H1-sh1TRα2 and 200 ng pCMV H1-sh2TRα2 vectors (shTRα2). A and B are samples from different animals. β actin was used as a loading control. B: TRα2 transient knockdown maintains T3-dependent activation of the positively regulated TyrH promoter. ShTRα2 has no effect on TyrH-luc transcriptional activity either in absence or presence of T3. shCt or shTRα2 (400 ng as above) were co-transfected with 1 µg of TyrH-luc construct/hypothalamus of hypothyroid 2-day old mice treated (PTU+T3) or not (PTU) by T3 (2.5 µg/g b.w.). C: TRα2 overexpression abrogates T3-independent repression of the positively regulated TyrH promoter. TRα2 overexpression significantly increases T3-independent TyrH-luc transcription as compared to Ct (p<0.001), but addition of T3 does not increase transcription further. Empty pSG5 vector (Ct) or pSG5-TRα2 (TRα2) was used at 100 ng and co-transfected with 1 µg of TyrH-luc construct/hypothalamus of hypothyroid 2-day old mice. D: TRα2 transient knockdown abolishes T3-dependent repression of the negatively regulated Trh promoter. ShTRα2 has no effect on T3-independent Trh promoter activity (p = 0.07) and when T3 is added, Trh-luc transcription is not repressed anymore (p<0.05) as compared to shCT. The same experimental conditions as in B were used (400 ng expression vector and 1 µg reporter gene, Trh-luc per pup). SEMs are given, n≥10 per point. In each case, the whole experiment was repeated twice giving similar results. *, p<0.05; **, p<0.01; ***, p<0.001.