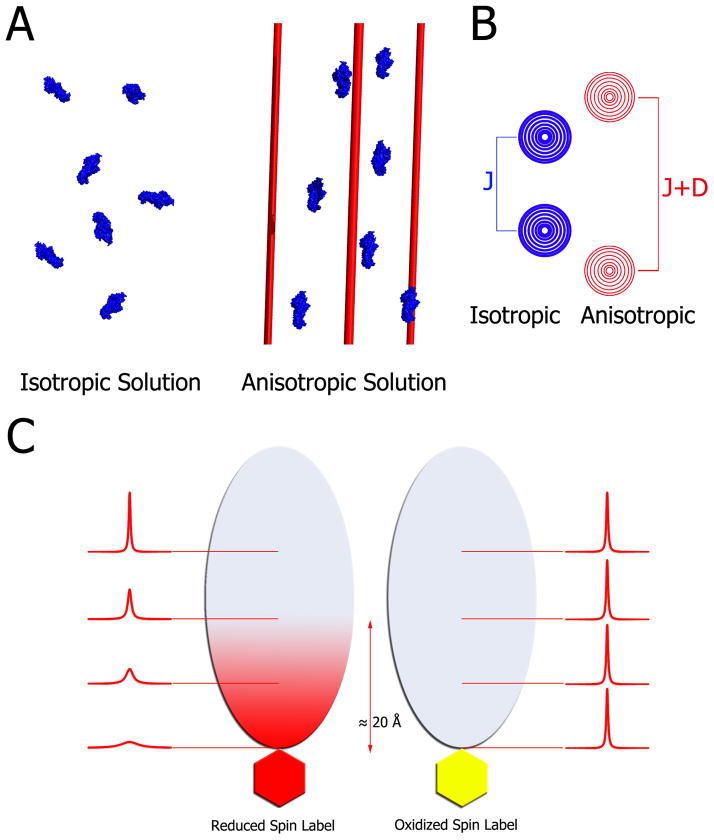
Figure 3. Principles of residual dipolar couplings and paramagnetic relaxation enhancement.
A) In an isotropic medium (left) proteins tumble freely averaging out any dipolar couplings. Addition of filamentous phage, nanotubes, bicelles, compressed gels etc. produces a modest anisotropy of the proteins (right). For illustration purposes all molecules here are aligned; however, in practice, the media are tuned to produce about 0.1% alignment. This partial alignment leads to an incomplete averaging of anisotropic magnetic interactions manifested in residual dipolar couplings. B) In isotropic conditions, the spectrum of a coupled resonance yields two peaks separated by the J-coupling. In anisotropic conditions, the peaks are separated by the sum of the J-coupling and the residual dipolar coupling. Therefore, RDCs can be easily extracted by comparing the effective couplings under isotropic and anisotropic conditions. C) For paramagnetic relaxation enhancement, a paramagnetic spin label, which is a stable free radical, is covalently linked to the thiol of a lone cysteine residue (left side). This spin-labeled protein is then added to 15N-labeled cognate partner, which subsequently broadens the interface resonances on its 1H-15N HSQC spectrum. Subsequently, the radical is quenched by reduction with ascorbic acid, and the spectrum is collected under the same conditions. Upon reduction, the previously broadened resonances reappear, which facilitates mapping of the interaction interface within ~20 Å of the spin-labeled probe.