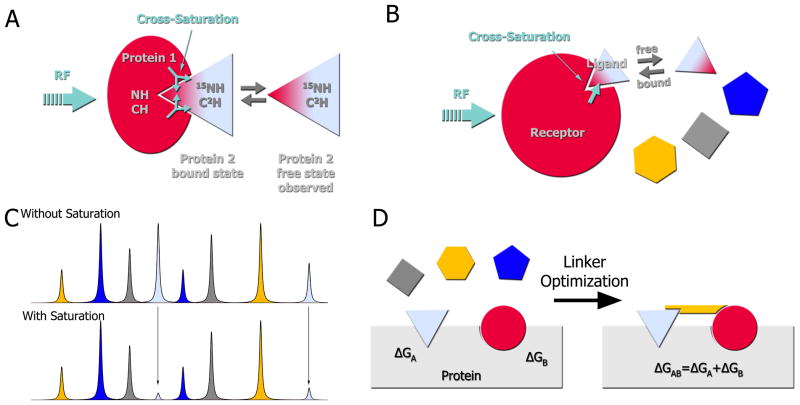
Figure 5. Cross-saturation transfer experiments for protein-protein complexes and drug discovery.
A) Principle of cross saturation transfer: The unlabeled receptor (protein 1) binds the 15N,2H-ligand (protein 2). The aliphatic frequencies of the receptor are then selectively irradiated, which then saturates the aliphatic, aromatic and amide resonances of the receptor through spin diffusion. This saturation is also transferred to ligand resonances located at the binding site and leads to a decrease in the respective HSQC peak intensities. If the complex has a high affinity, the HSQC spectrum would show the peaks of the bound form of the ligand. If the complex is weak, the HSQC would show the spectrum of the free ligand; nonetheless, the saturation has a longer lifetime than the exchange rate between free and bound form and the free form still “remembers” the saturation. B) Application of the cross saturation transfer principle to a mixture of fragments. The same principle as described in A) applies to any ligand binding to the receptor. C) In a mixture of compounds, only fragments that bind to the receptor (gray compound) would exhibit a change in peak intensities. D) Illustration of the SAR by NMR method [35]. First, a fragment library is screened against a protein of interest until two compounds, A and B, are identified that can simultaneously bind to proximal sites. These compounds are then linked together and again screened against the protein. Theoretically, these compounds can be linked into a single molecule whose binding affinity is the summation of the individual binding energies.