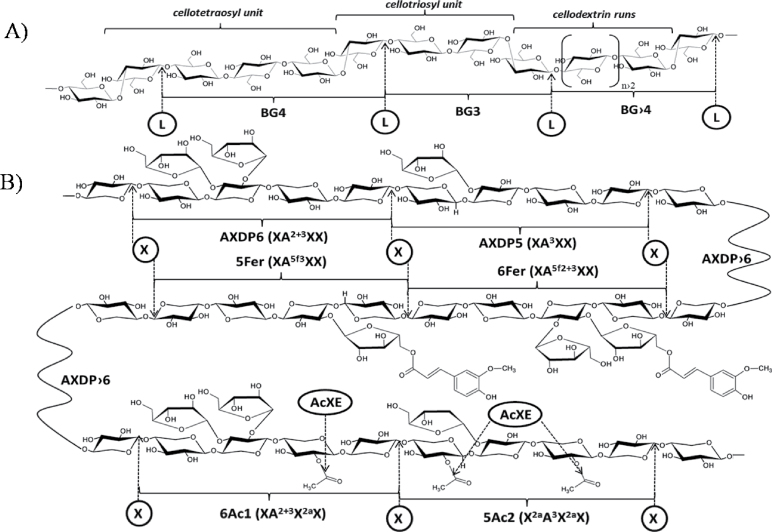
Fig. 1.
Structure of β-glucans (A) and arabinoxylans (B). Bonds that are susceptible to hydrolysis by the enzymes used in this study (L, lichenase; X, xylanase; AcXE, acetylxylan esterase) are marked with arrows, and main oligomers released by enzymic digestion are labelled as in the manuscript. Arabinoxylan (AX) oligomers are also abbreviated according to the Fauré et al. (2009) nomenclature for heteroxylans (in parentheses): starting position is nonreducing d-xylosyl unit; X, unsubstituted xylosyl residue; A, xylosyl residue substituted by l-arabinofuranosyl residue; a, acetyl residue; f, feruloyl residue; 2, (1→2) linkage; 3, (1→3) linkage; 5, (1→5) linkage. Note that lichenase specifically cleaves the β (1→4) linkage of 3-O-substituted d- glucopyranosyl residue in β-glucans (BG) and xylanase specifically attacks β (1→4) glycosidic bonds between two unsubstituted xylopyranosyl residues in AX.