Abstract
Transient elastography (TE) has been used as a non-invasive method for liver stiffness measurement (LSM) in patients with chronic liver disease. This study was performed to assess the change of LSM by TE and to assess its clinical usefulness during long-term oral antiviral therapy in patients with chronic hepatitis B (CHB). We retrospectively reviewed 83 CHB patients. The mean interval between two LSM was 411.5 ± 149.5 days. Initial and follow-up LSM was 16.15 ± 12.41 kPa and 11.26 ± 7.36 kPa, respectively (P < 0.001). The degree of regression of liver stiffness was -2.03 ± 0.36% per month. The fibrosis stage classified by LSM value improved in 37 (44.6%) patients during oral antiviral therapy. Of the 30 (36.1%) patients with LSM ≥ 14.1 kPa (cirrhosis) at 1st LSM, 12 (40%) proved to no longer have cirrhosis (≥ 1 decrease in fibrosis stage) at 2nd LSM. LSM significantly decreased in both baseline high (> upper limit of normal [ULN] × 2) and low (≤ ULN × 2) alanine aminotransferase groups during antiviral therapy (P < 0.001; P = 0.001, respectively). Long-term oral antiviral therapy resulted in the improvement of liver stiffness in a substantial portion of patients with CHB. TE may be used a useful clinical tool to assess disease progression in CHB patients.
Graphical Abstract
Keywords: Elasticity Imaging Techniques; Liver Cirrhosis; Hepatitis B, Chronic; Liver Stiffness; Oral Antiviral Agents
INTRODUCTION
Chronic hepatitis B (CHB) often leads to serious health conditions including decompensated liver cirrhosis and hepatocellular carcinoma (HCC) (1). Host immune response to HBV infected hepatocytes results in hepatocyte apoptosis and necrosis that activates nonparenchymal cells including hepatic stellate cells to produce extracellular matrix such as type I collagen resulting in hepatic fibrosis (2, 3). Hepatic fibrogenesis is a dynamic process with a net result of fibrotic scar formation. Resolution of scars determines the progression from hepatic fibrosis to liver cirrhosis (4). The suppression of viral replication by long-term antiviral therapy may improve hepatic inflammation and fibrosis and halt the progression of disease to decompensated liver cirrhosis and HCC (5, 6). Lamivudine treatment decreased complications from portal hypertension and the development of liver cancer in patients with compensated liver cirrhosis, although the benefit was less in patients who develop lamivudine resistance (7). Entecavir treatment for 48 weeks in patients with advanced hepatic fibrosis or cirrhosis showed the improvement of liver histology in 57% with HBeAg+ve, 59% with HBeAg-ve, and 43% of patients with lamivudine-resistant CHB (8).
Accurate information about the hepatic fibrosis stage is very important in clinical practice because the extent of hepatic fibrosis predicts the development of hepatic decompensation and HCC in patients with CHB (9, 10). Liver biopsy has been a gold standard to assess the extent of hepatic fibrosis (11). However it is hard to perform liver biopsy repeatedly in patients with CHB because of its invasive nature. Instead, the assessment of liver stiffness using fibroscan has been reported as a relevant non-invasive tool for the assessment of hepatic fibrosis in various kinds of chronic liver disease including hepatitis B, hepatitis C, and alcoholic liver disease (12). In this study we aimed to evaluate the regression of hepatic fibrosis through repeated liver stiffness measurement (LSM) using fibroscan during long-term oral antiviral therapy in patients with CHB.
MATERIALS AND METHODS
Patients
From October 2007 to February 2011, a total of 3,156 LSM was performed in Gangnam Severance Hospital, Seoul, Korea. Eighty eight CHB patients on oral nucleos(t)ide therapy who received LSM twice were retrospectively enrolled. Five patients who had the interval between the first and the second LSM was shorter than 6 months, were excluded. No patients were positive to anti-hepatitis C virus (HCV) antibody (Ab) or anti-human immunodeficiency virus Ab. Finally, 83 patients were included in this study. We retrospectively reviewed the medical records and analysed the change of liver stiffness measured by transient elastography (Fibroscan®, Echosens, France). The patients were followed up every 3 months with serologic tests. When virologic breakthrough due to the development of antiviral drug resistance was confirmed, nucleos(t)ide analogue was added or changed according to resistant mutation.
Laboratory tests
HBsAg and HBeAg/Ab were determined by enzyme immunoassay (Dade Behring, Marburg, Germany). Serum HBV DNA was measured by a real-time polymerase chain reaction assay using a COBAS TaqMan 48 analyzer (Roche Diagnostics, Mannheim, Germany) with a detection limit of 12 IU/mL. The antibody against HCV was detected by a third-generation enzyme-linked immunosorbent assay (Korea Greencross, Yong-in, Korea).
Liver stiffness measurement (LSM)
Well trained and experienced physicians obtained all LSM according to the instructions provided by the manufacturer. All patients received LSM using FibroScan® two times during oral nucleos(t)ide antiviral therapy. The first LSM was performed before the starting antiviral therapy (n=33, 39.8%) or during follow up visit on antiviral therapy (n=50, 60.2%). The timing of the 2nd LSM was decided by physician's or patient's discretion to monitor liver stiffness. The mean interval between the first and the second LSM was 411.5±149.5 (range: 180-1,062) days. LSM was performed on the right lobe of the liver through the intercostal spaces on patients lying in the dorsal decubitus position with the right arm in maximal abduction (13). The results are expressed as kilopascals (kPa). The extent of liver fibrosis was classified according to the METAVIR scoring system (F0-F4). Transient elastography cut-off levels used for staging liver fibrosis ≥F2, ≥F3, and F4 were 8.8, 10.2, and 14.1 kPa, respectively (14).
The interquartile range (IQR) was defined as an index of intrinsic variability of LSM corresponding to the interval of LSM results containing 50% of the valid measurements between the 25th and 75th percentiles. The median LSM was selected as representative of the LSM value in a given patient. In this study, only LSM examinations with at least ten validated measurements, an IQR to median value ratio (IQR/M) was less than 0.3, and a success rate of at least 60% were considered reliable and were included for the analysis.
Statistics
Data were analyzed using the statistical software SPSS (version 13.0, SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as mean±standard deviation. The degree of difference of LSM between two time points (% per month) was calculated as follows;
Data were analyzed using paired t-test, Wilcoxon signed rank test, Mann-Whitney U test, oneway ANOVA, and chi-square test. Data were considered to be statistically significant with P < 0.05.
Ethics statement
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board at Gangnam Severance Hospital, Seoul, Korea (3-2013-0269). Informed consents were waived by the board.
RESULTS
Baseline characteristics of the patients
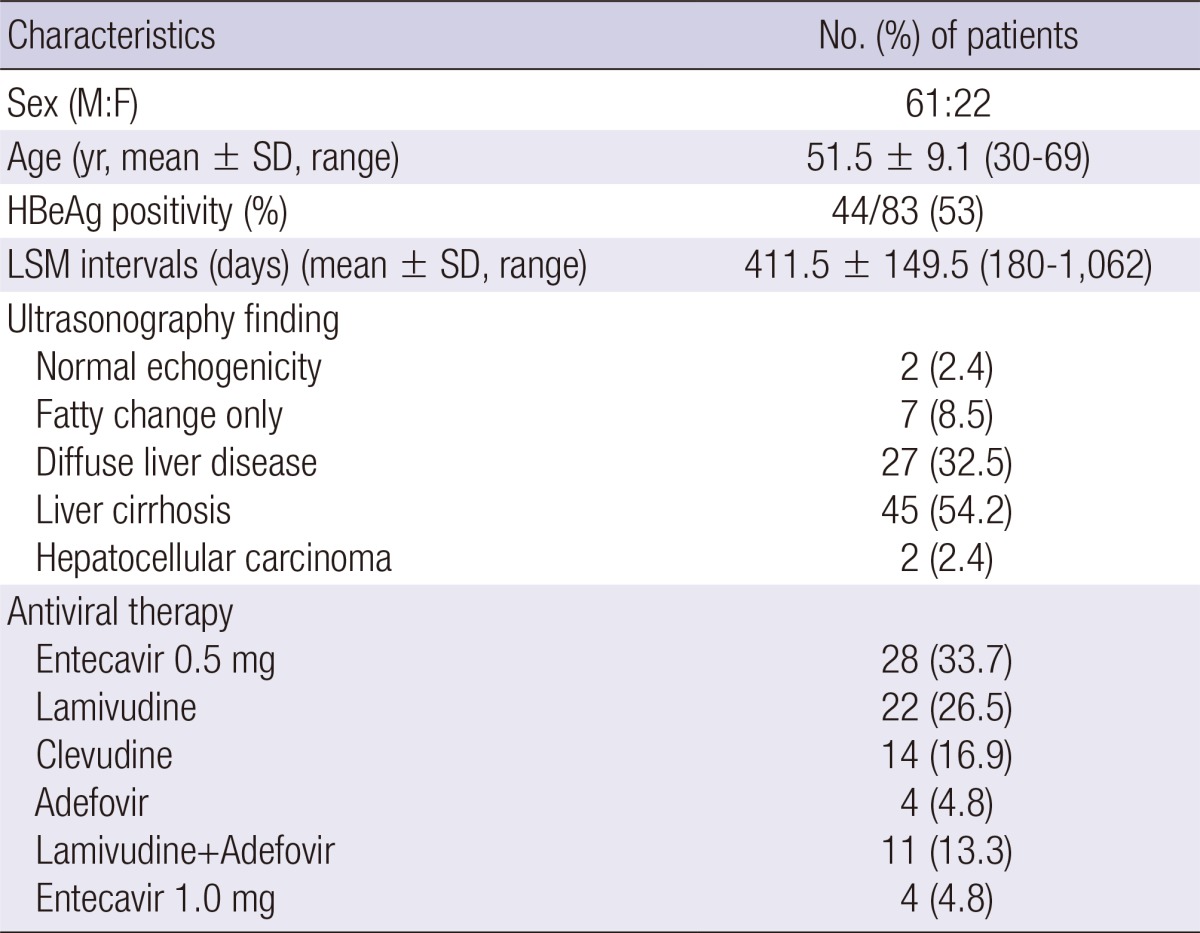
The baseline characteristics of the 83 patients are presented in Table 1. There were 61 men and 22 women, with a mean age of 51.5±9.1 yr. The mean interval between two LSMs was 411.5 ±149.5 (180-1,062) days. The number of patients received antiviral therapy as follows; entecavir 0.5 mg in 28 (33.7%), lamivudine in 22 (26.5%), clevudine in 14 (16.9%), adefovir in 4 (4.8%), lamivudine plus adefovir in 11 (13.3%), and entecavir 1.0 mg in 4 (4.8%) patients. Eighty patients (96.4%) showed serum HBV DNA below 2,000 IU/mL at 2nd LSM.
Table 1.
Baseline characteristics of patients
Comparison of clinical parameters between two LSMs during antiviral therapy
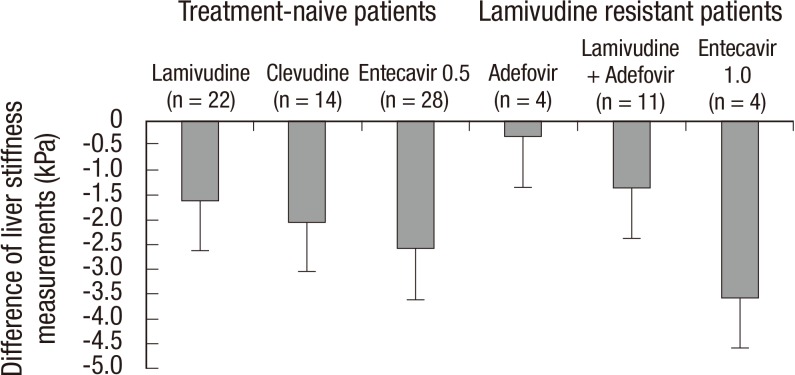
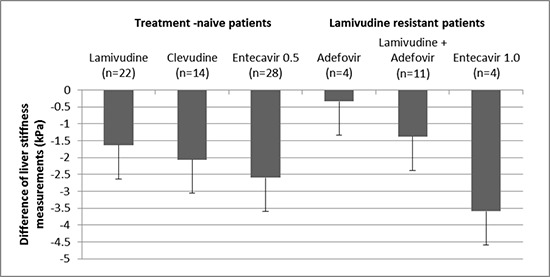
Clinical parameters and liver stiffness values are demonstrated in Table 2. During the antiviral therapy, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and HBV DNA levels were decreased. Accordingly, serum albumin levels and platelet count increased during the antiviral therapy. Liver stiffness values significantly decreased from 16.15± 12.41 kPa to 11.26±7.36 kPa during the antiviral therapy (P < 0.001). Sixty-two (74.7%) patients had a lower liver stiffness value at 2nd LSM compared to 1st LSM. The degree of regression of liver stiffness value was -2.03±0.36 (-10.25~8.83)% per month. There was no difference of the degree of regression of liver stiffness between the patients in whom initial LSM was obtained before starting antiviral therapy (n=33) and the patients in whom initial LSM was obtained during antiviral therapy (n=50) (-2.54 ±3.13 vs -1.69±3.35% per month, P =0.251). The difference of liver stiffness value between 1st and 2nd LSMs by the type of antiviral agents is summarized in Fig. 1. The differences of two LSM values were all negative regardless of antiviral agents. However, there was no significant difference according to the kind of antiviral agents (P = 0.625) or lamivudine resistance (P = 0.078).
Table 2.
Comparison of parameters between two LSMs during antiviral therapy
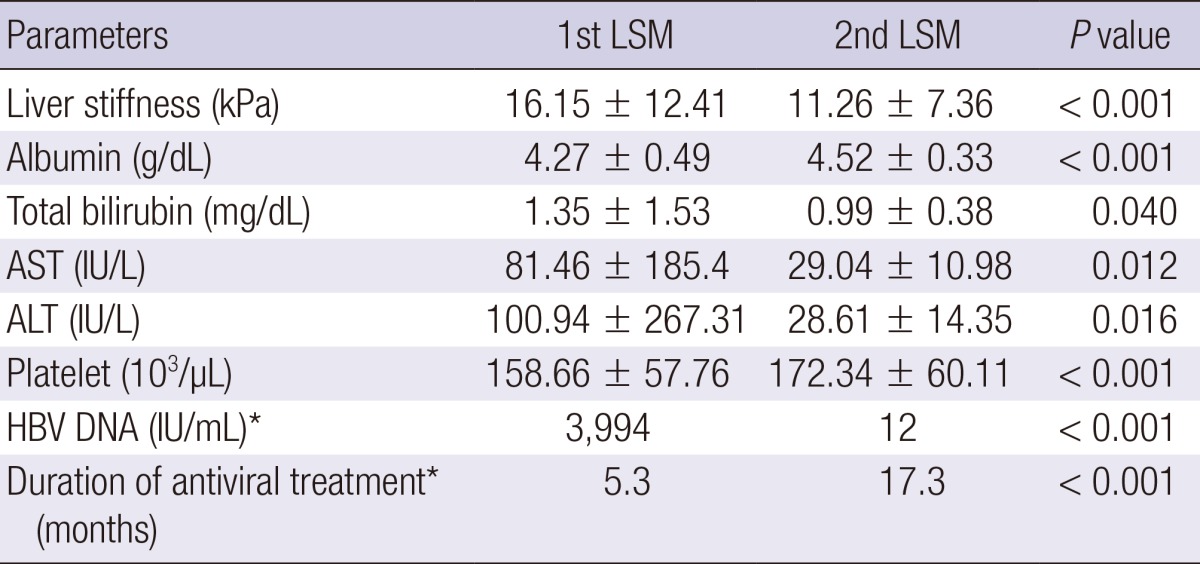
*Median. AST, aspartate aminotransferase; ALT, alanine aminotransferase; LSM, liver stiffness measurement.
Fig. 1.
The difference of liver stiffness values between the first and the second measurement using transient elastography (Fibroscan®) by the type of antiviral agents. The differences of two LSM values were all negative values regardless of antiviral agents. However, there was no significant difference according to the kind of antiviral agents (P = 0.625) or lamivudine resistance (P = 0.078).
Of the 30 (36.1%) patients with LSM≥14.1 kPa (cirrhosis) at 1st LSM, 25 (83.3%) had a lower LSM value and 12 (40%) proved to no longer have cirrhosis (≥1 decrease in fibrosis stage) at 2nd LSM.
Comparison of groups according to the change of LSM value during antiviral therapy
We compared the clinical parameters according to the presence of the improvement of LSM value during antiviral therapy (Table 3). Sixty two (74.7%) patients had a decrease in liver stiffness value (group 1), whereas 21 (25.3%) showed an increase in liver stiffness value (group 2) during antiviral therapy. There was no difference in sex, age, LSM intervals, baseline laboratory profiles including AST, ALT, total bilirubin, HBeAg positivity, HBV DNA levels between two groups. Only 1st LSM value was higher in group 1 (P = 0.012), and 2nd LSM value was lower in group 1 compared to group 2 (P = 0.005).
Table 3.
Comparison of groups by LSM change during antiviral therapy
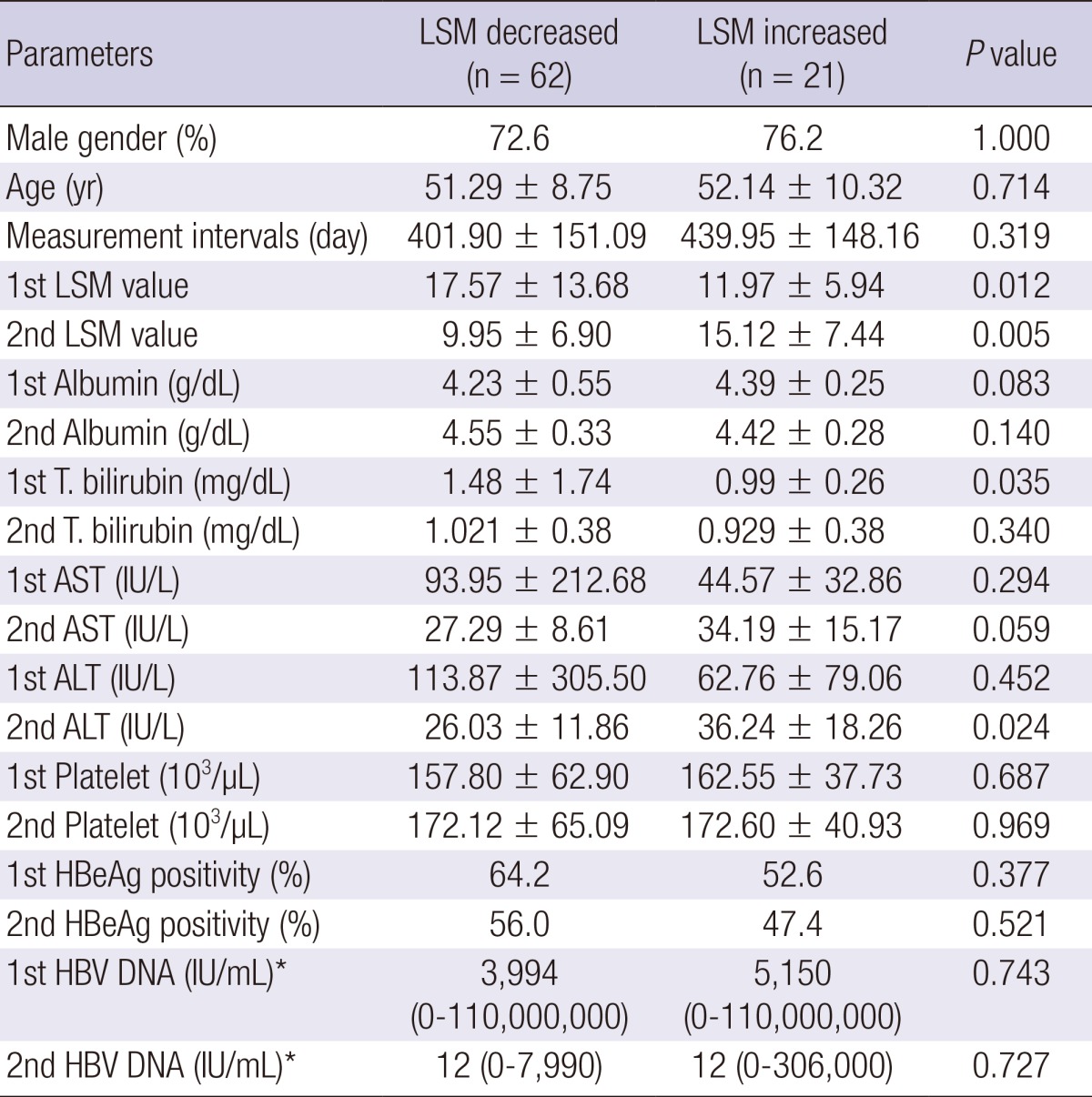
*Median. AST, aspartate aminotransferase; ALT, alanine aminotransferase; LSM, liver stiffness measurement; HBeAg, hepatitis B envelop antigen; HBV, hepatitis B virus.
Comparison of groups according to the change of fibrosis stage during antiviral therapy
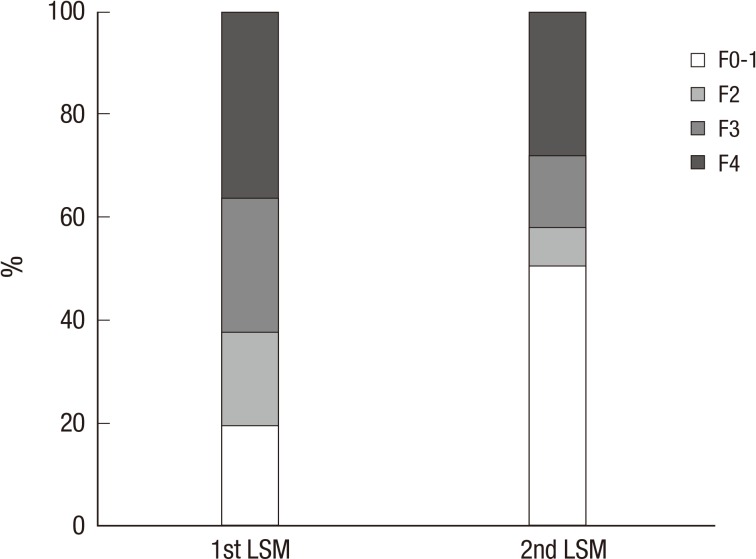
The patients' initial fibrosis stages were 16 (19.3%) in F0-1, 15 (18.1%) in F2, 22 (26.5%) in F3 and 30 (36.1%) in F4, respectively. After antiviral therapy, the patients' fibrosis stages were 42 (50.6%) in F0-1, 6 (7.2%) in F2, 12 (14.5%) in F3 and 23 (27.7%) in F4, respectively (Fig. 2). The fibrosis stage was improved in 37 (44.6%) between two LSMs. Thirty eight (45.8%) patients showed no change of fibrosis stage, only 8 (9.6%) showed the aggravation of fibrosis stage. Among these 8 patients, their initial ultrasonographic findings were diffuse liver disease in 2 patients, and liver cirrhosis in 6 patients. The antiviral treatment for these 8 patients was as follows; lamivudine for 2 patients, clevudine for 2 patients, entecavir for 2 patients, adefovir for one patient, and lamivudine and adefovir combination for one patient. Clinical parameters according to the improvement of fibrosis stage were compared (Table 4). There was no difference in age, LSM intervals, initial laboratory profiles including AST, ALT, total bilirubin, HBeAg positivity, and HBV DNA levels. However, patients with improved fibrosis stage were female predominant, and had lower initial LSM value and higher initial albumin level.
Fig. 2.
Changes of the proportion of the patients grouped by the fibrosis stage between two liver stiffness measurements during oral antiviral therapy. The patients' initial fibrosis stages were 16 (19.3%) in F0-1, 15 (18.1%) in F2, 22 (26.5%) in F3 and 30 (36.1%) in F4, respectively. After antiviral therapy, the patients' fibrosis stages were 42 (50.6%) in F0-1, 6 (7.2%) in F2, 12 (14.5%) in F3 and 23 (27.7%) in F4, respectively.
Table 4.
Comparison of groups by fibrosis stage improvement during antiviral therapy
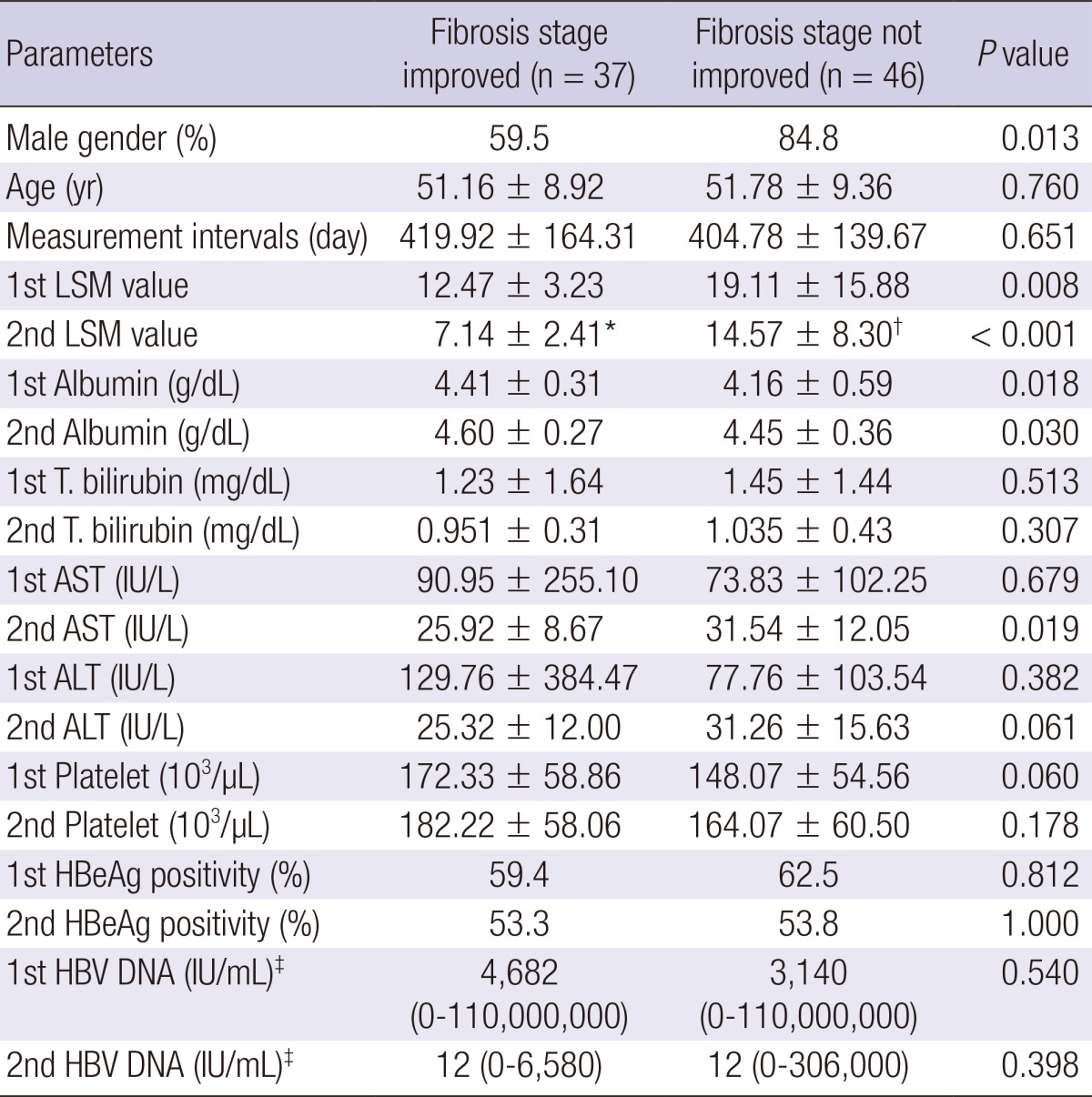
*P < 0.001; †0.011 compared to 1st LSM values; ‡Median. AST, aspartate aminotransferase; ALT, alanine aminotransferase; LSM, liver stiffness measurement; HBeAg, hepatitis B envelop antigen; HBV, hepatitis B virus.
Comparison of groups according to ALT levels at 1st LSM during antiviral therapy
It has been reported that ALT level may affect the LSM value. So we compared two groups accoding to ALT levels at 1st LSM time point (Table 5). AST, ALT, and HBV DNA levels were significantly higher in the high ALT (>upper limit of normal [ULN]×2) group. However, there was no difference in sex, age, LSM measurement intervals, baseline albumin, total bilirubin, and HBeAg positivity between two groups. The second LSM significantly decreased in both high ALT (>ULN×2) and low ALT (≤ULN ×2) group compared to 1st LSM (P < 0.001; P=0.001, respectively).
Table 5.
Comparison of groups by ALT levels at 1st LSM during antiviral therapy
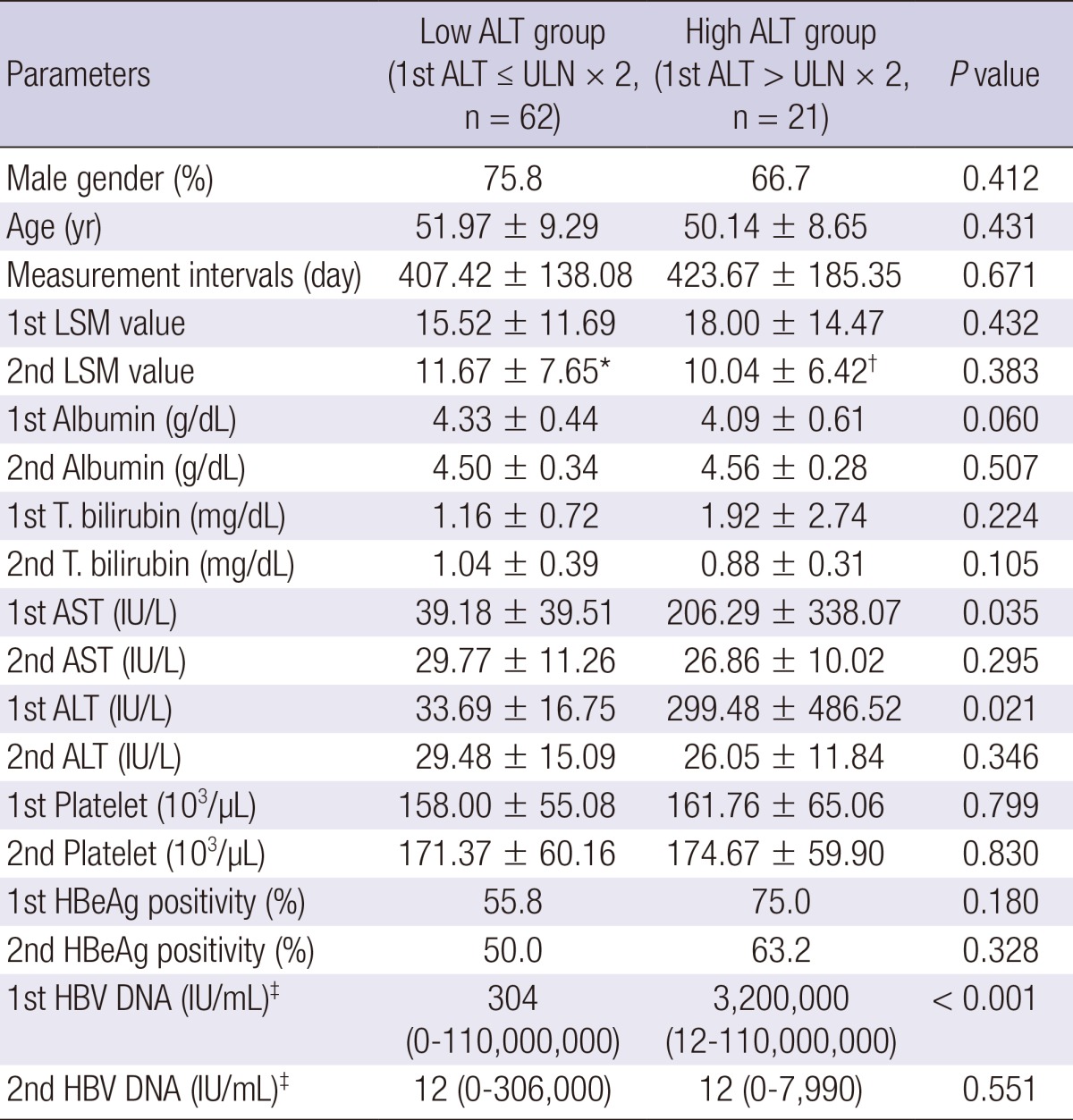
*P < 0.001; †0.001 compared to 1st LSM values; ‡Median. AST, aspartate aminotransferase; ALT, alanine aminotransferase; LSM, liver stiffness measurement; HBeAg, hepatitis B envelop antigen; HBV, hepatitis B virus.
DISCUSSION
Chronic HBV infection is one the most common etiologies of chronic liver disease in Korea (15). There are several clinical features of HBV-related chronic liver diseases in Korean population (16). HBV genotype C is more than 95%, and precore and core promoter mutant HBV are common. Lamivudine resistance is not uncommon among CHB patients who are candidates for the treatment with oral nucleos(t)ide analogues in Korea. The aim of treating patients with chronic HBV infection is to prevent disease progression to liver cirrhosis and hepatocellular carcinoma (17). Oral antiviral agents are widely used to suppress viral replication and to prevent disease progression. However, long-term antiviral therapy is usually required because of the nature of oral nucleos(t)ide analogues. After introduction of potent antiviral agents such as entecavir or tenofovir, it becomes possible to maintain HBV viral load very low or even undectable during long-term antiviral therapy. Moreover, the reversal of hepatic fibrosis/cirrhosis was observed in patients with long-term oral antiviral therapy (6).
Liver fibrosis results from wound healing response for chronic inflammation in the liver. The stage of liver fibrosis has a good correlation with liver-related clinical outcome such as decompensated liver cirrhosis or even development of HCC (18, 19). The accurate information about the extent of liver fibrosis in patients with CHB is clinically very important for the evaluation of severity of the disease, for making treatment decisions and for evaluating efficacy of antiviral treatment. Liver biopsy has long been a gold standard to assess the degree of liver fibrosis (11). However, because of invasive nature and risk of bleeding complication it is difficult to perform liver biopsy, particularly when it needs to be repeated in real clinical practice. Moreover, liver biopsy has limitations such as extremely small sampling size representing only 1/50,000 liver volume, intra- or inter-observer variation for the accurate assessment of liver fibrosis (20).
Recently noninvasive methods such as transient elastograpy, acoustic radiation force impulse (ARFI), Fibro-CT, MRI have been explored for the assessment of hepatic fibrosis (12). Liver stiffness measurement by fibroscan is the most widely used noninvasive method for assessing the extent of liver fibrosis. It was reported that LSM discriminates significant liver fibrosis or liver cirrhosis (F4) with high accuracy in patients with chronic viral hepatitis (21, 22). Our study showed that mean LSM was decreased from 16.15±12.41 kPa to 11.26±7.36 kPa during antiviral therapy for an average 411 days, suggesting the regression of hepatic fibrosis during oral antiviral therapy. The average of degree of regression of liver stiffness during antiviral therapy was -2.03±0.36% per month. There was no statistical difference of degree of regression by the type of antiviral agents or presence of lamivudine resistance. These results might be caused by the small number of patients in each oral antiviral agents group, and should be studied further in a larger study.
The heterogeneous timing of LSM from the retrospective study design is a limitation. The 1st LSM was measured as a baseline before starting antiviral therapy in 33 patients (39.8%). Median duration of antiviral treatment to 1st and 2nd LSM time point was 5.3 and 17.3 months, respectively. There was no difference of the degree of regression of liver stiffness between the groups in whom initial LSM was obtained before starting antiviral therapy (n=33) and the patients in whom initial LSM was obtained during antiviral therapy (n=50) (-2.54±3.13 vs -1.69±3.35% per month, P = 0.251). Therefore it is less likely that the improvement of LSM mainly resulted from lead time bias by previous long-term antiviral therapy.
In our study, we adopted LSM cut-offs for fibrosis staging from a previous report in which Korean patients with genotype C HBV infection were studied. Of the 30 (36.1%) patients with LSM ≥14.1 kPa (F4) at 1st LSM, 12 (40%) proved to have improved fibrosis (LSM less than 14.1 kPa) at 2nd LSM. According to recent meta-analysis, weighted mean LSM values were 7.9, 8.8, and 11.7 for F≥2, F≥3, and F=4 (23). When we apply cut-offs 7.9, 8.8, and 11.7 kPa for F≥2, F≥3, and F=4 from the meta-analysis, LSM improvement was also observed in a very similar manner during antiviral therapy compared with our current data. The fibrosis stage was improved in 39 (47.0%) between two LSMs. Thirty six (43.4%) patients showed no change of fibrosis stage, only 8 (9.6%) showed the aggravation of fibrosis stage. Of the 39 (47.0%) patients with LSM ≥11.7 kPa (cirrhosis) at 1st LSM, 17 (43.6%) proved to no longer have cirrhosis (≥1 decrease in fibrosis stage) at 2nd LSM. Taken together, these data indicate that oral antiviral therapy attenuates the extent of liver fibrosis in patients with B-viral liver cirrhosis.
For the assessment of hepatic fibrosis by LSM using Fibroscan, the following important limitation should be considered. The degree of hepatic necroinflammation may affect the LSM value, i.e., the presence of significant necroinflammation can exaggerate the LSM value (24, 25, 26). When we analysed the change of LSM during antiviral therapy with respect to baseline ALT level, the LSM value significantly decreased in high ALT (> ULN×2) group as well as in low ALT (≤ ULN×2) group, indicating that oral antiviral therapy decrease liver stiffness regardless of ALT abnormality in patients with CHB. These results suggest that the decrease in LSM value during oral antiviral therapy is not caused by purely reducing hepatic necroinflammation but at least partially by decreasing hepatic fibrosis. Macronodular cirrhotic architecture or the absence of significant histologic grade may results in false negative LSM (LSM below cutoff level of cirrhosis) for the accurate diagnosis of histologic cirrhosis. The absence or mild histologic grade during oral antiviral therapy in our study might cause lower 2nd LSM value resulting in the underestimation of liver cirrhosis.
Because of retrospective study design, pathologic changes were not verified to confirm the change of LSM in our study. In spite of this limitation, the demonstration of the LSM change in CHB patients on antiviral therapy through repeated measurement of liver stiffness in real life setting provides important clinical implication supporting the usefulness of LSM using Fibroscan for the assessment of fibrosis regression during antiviral treatment. Recently, Marcellin et al. demonstrated that oral antiviral treatment with tenofovir regressed liver cirrhosis in chronic hepatitis B patients (27). Our study provides similar results using repeated LSM measurement instead of repeated liver biopsies in real-life practice setting. Our study result warrants further prospective study to confirm the correlation of LSM and pathologic changes during oral antiviral therapy in CHB patients.
In conclusion, long-term oral antiviral therapy resulted in the improvement of liver stiffness in a substantial portion of patients with CHB. Liver stiffness measurement using transient elastography may be used as a useful clinical tool to evaluate disease progression in CHB patients.
Footnotes
The authors have no conflict of interest to disclose.
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
References
- 1.Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797–816. doi: 10.1016/j.cld.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21:S84–S87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 5.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 6.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 8.Schiff E, Simsek H, Lee WM, Chao YC, Sette H, Jr, Janssen HL, Han SH, Goodman Z, Yang J, Brett-Smith H, et al. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol. 2008;103:2776–2783. doi: 10.1111/j.1572-0241.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 9.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Guido M, Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89–97. doi: 10.1055/s-2004-823103. [DOI] [PubMed] [Google Scholar]
- 12.Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 13.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim BK, Kim SU, Kim HS, Park JY, Ahn SH, Chon CY, Cho IR, Joh DH, Park YN, Han KH, et al. Prospective validation of FibroTest in comparison with liver stiffness for predicting liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e35825. doi: 10.1371/journal.pone.0035825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH. Trends in the seroprevalence of hepatitis B surface antigen in the South Korean population. Int J Infect Dis. 2012;16:e669–e672. doi: 10.1016/j.ijid.2012.05.1019. [DOI] [PubMed] [Google Scholar]
- 16.Yoo BC, Park JW, Kim HJ, Lee DH, Cha YJ, Park SM. Precore and core promoter mutations of hepatitis B virus and hepatitis B e antigen-negative chronic hepatitis B in Korea. J Hepatol. 2003;38:98–103. doi: 10.1016/s0168-8278(02)00349-5. [DOI] [PubMed] [Google Scholar]
- 17.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 19.Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan) Hepatology. 2011;53:885–894. doi: 10.1002/hep.24121. [DOI] [PubMed] [Google Scholar]
- 20.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. doi: 10.1371/journal.pone.0044930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim SU, Seo YS, Cheong JY, Kim MY, Kim JK, Um SH, Cho SW, Paik SK, Lee KS, Han KH, et al. Factors that affect the diagnostic accuracy of liver fibrosis measurement by Fibroscan in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2010;32:498–505. doi: 10.1111/j.1365-2036.2010.04353.x. [DOI] [PubMed] [Google Scholar]
- 26.Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 27.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]