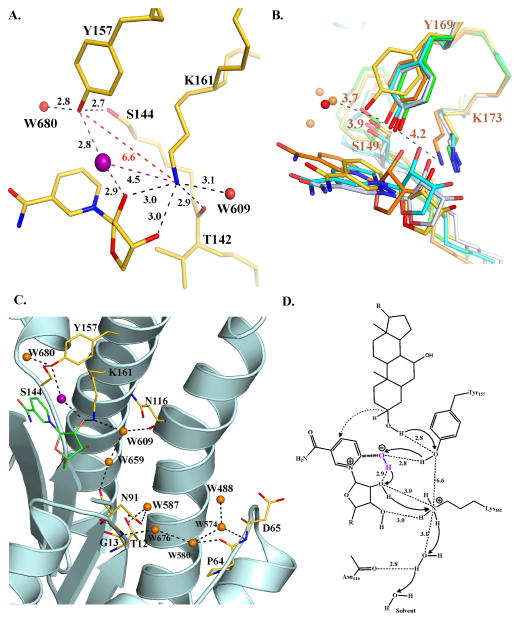
FIGURE 5.
Active site of BaiA2 and proposed catalytic mechanism. (A) Orientation of the active site residues. Dashed black colored lines indicate hydrogen bonding interactions. Non-hydrogen bonding interactions are indicated in dashed red lines. Numbers denote distances in Å. The O atom of OH−/water of the nicotinamide-OH− adduct is depicted as a purple sphere. (B) Comparison of the interaction between active site residues of BaiA2, MtDh (NADP-; PDB Code: 1H5Q), 3α, 20β-hydroxysteroid dehydrogenase (NAD-; PDB Code: 2HSD), 3β, 17β-hydroxysteroid dehydrogenase (apo-; PDB Code: 1HXH), and Rv2002 (ternary-; PDB Code: 1NFQ). Water molecules in BaiA2 and MtDh are depicted as red and orange spheres, respectively. Numbers indicate distances in Å from the terminal functional groups of Tyr169 and Lys173 of MtDh. (C) Proton relay network observed in BaiA2:NAD+ structure. O atoms of protein residues and bound water molecules are colored red and orange, respectively. Dashed black colored lines depict hydrogen bonding interactions. (D) Proposed mechanism of oxidation catalyzed by BaiA1/A2. Catalysis initiated by proton abstraction from Tyr157-OH group by nicotianmide-OH− adduct (denoted in purple). The terminal oxyanion of Tyr157 is re-protonated by the proton from the C3-hydroxyl group of the substrate and hydride transfer (dashed arrow) from C3 atom of the substrate to the C4 atom of the nicotinamide moiety. The proton abstracted by the nicotinamide-hydroxyl adduct is relayed through the 2′-hydroxyl group of nicotinamide ribose, Lys161 and a solvent channel denoted in panel C. Solid black arrows indicate direction of proton relay. Numbers indicate distances in Å observed in BaiA2:NAD+ crystal structure. The denoted distance of C3-OH group of the substrate from Tyr157-OH is based on the observed distance of water molecule, W680, in BaiA2:NAD+ structure that might represent the 3-oxygen atom of the substrate. In figures A, B, and C, C atoms of protein residues are colored gold (BaiA2), green (3β, 17β-hydroxysteroid dehydrogenase), cyan (3α, 20β-hydroxysteroid dehydrogenase), orange (MtDh) and gray (Rv2002). N atoms are colored blue. For clarity only the nicotinamide and the linked ribose moiety of NAD+ are shown. Oxygen atom of the nicotinamide-OH−/water adduct is denoted by purple sphere in figures A and C.