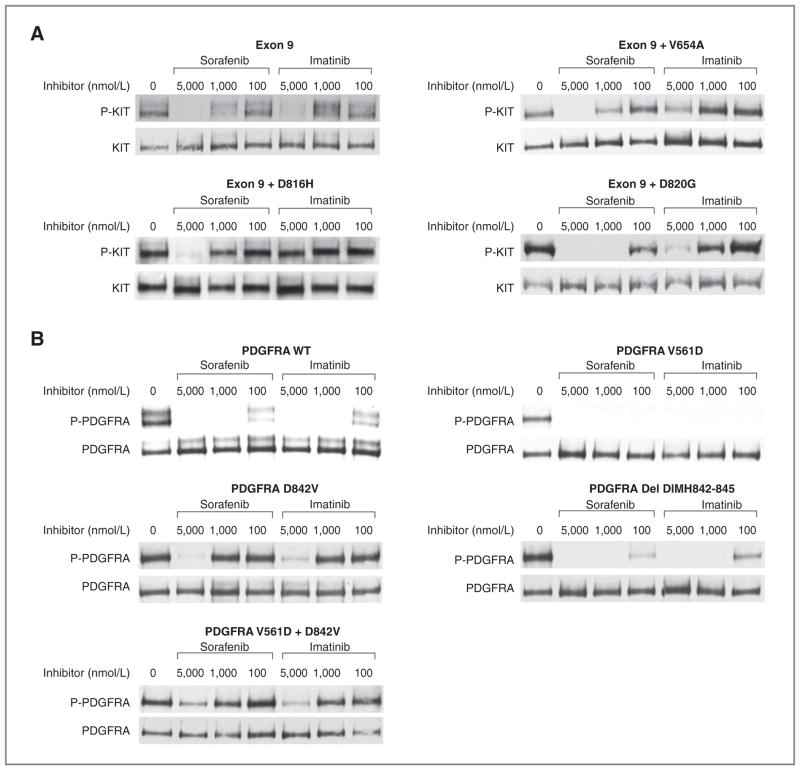
Figure 2.
Comparative biochemical activity of imatinib versus sorafenib for extracellular KIT mutants or PDGFRA-mutant kinases in transiently transfected cells. CHO cells were transiently transfected and protein lysates from transfected cells were prepared and subjected to immunoprecipitation using anti-KIT or anti-PDGFRA antibodies followed by sequential immunoblotting for phospho-KIT (P-KIT) or total KIT (KIT), or phosphotyrosine (P-PDGFRA) or total PDGFRA (PDGFRA), respectively, as previously reported. A, representative results from a minimum of 3 replicated experiments of the comparative activity of imatinib or sorafenib for inhibiting the isolated KIT exon 9 mutation insertion 502–503 AY or compound mutations of KIT exon 9 with a secondary imatinib-resistant mutation are shown. B, representative results from a minimum of 3 replicate experiments of the comparative activity of imatinib or sorafenib for inhibiting WT (unmutated, PDGF-AA ligand-stimulated PDGFRA), isolated PDGFRA exon 12 (V561D) or exon 18–mutant kinases (D842V, deletion DIMH842-845), or the compound mutant kinase V561D + D842V are shown.