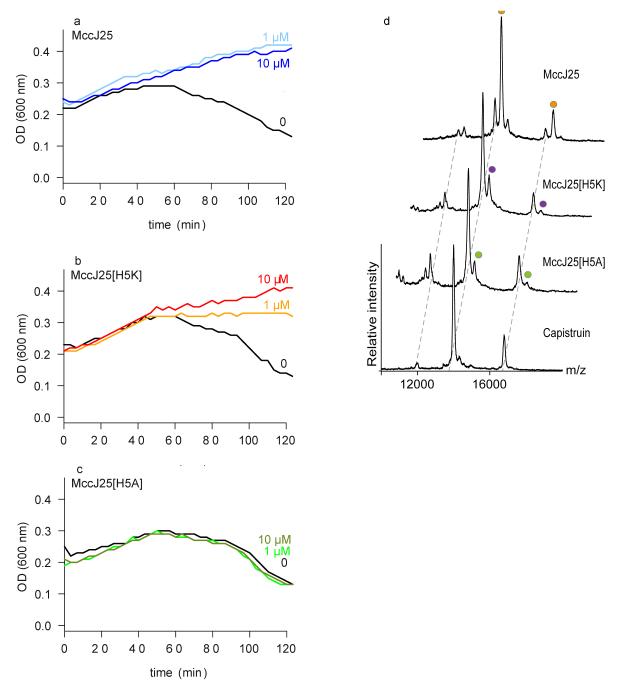
Fig 2.
Interaction studies of lasso peptides with FhuA by phage T5 infection and non-denaturing mass spectrometry. (a-c) E. coli W3110 was incubated with MccJ25 (a), MccJ25H5K (b), and MccJ25H5A (c) at concentrations of 1 or 10 μM, or with solvent only. After addition of phage T5, lysis was monitored by measuring culture turbidity (OD 600 nm) for 120 min. The data shown are the average of three measurements for each condition. (d) Non-denaturing mass spectra showing binding of MccJ25, MccJ25H5K and MccJ25H5A to FhuA. Increasing concentrations of the peptides were titrated to FhuA (Supplementary Fig. 5). Only the 7.5 μM peptide concentration of the MccJ25 and its variants are shown. Cap is shown at 20 μM peptide concentration. Each mass spectrum and mass-to-charge peak is assigned according to incubation condition. In the mass spectra we observed higher molecular weight species with low intensity that probably corresponded to non-specific binding of a second peptide at highest concentration; a second binding molecule was also observed from the ITC data 7.