Abstract
Structured Abstract
Objectives
To investigate the association between resting myocardial function as assessed by tissue Doppler myocardial velocities (TDI) and the propensity for developing mental stress induced ischemia (MSIMI).
Background
Tissue Doppler myocardial velocities detect preclinical cardiac dysfunction and clinical outcome in a range of conditions. However, little is known about the interrelationship between myocardial velocities and the propensity for developing MSIMI versus exercise stress induced myocardial ischemia (ESIMI).
Methods
Resting annular myocardial TDI velocities were obtained in 225 patients with known coronary heart disease who were subjected to both conventional exercise stress test as well as a battery of 3 mental stress tests. Diastolic early (e′) and late (a′) as well as systolic (s′) velocities were obtained and eas-index, an integrated measure of myocardial velocities, was calculated as e′/(a′ x s′). MSIMI was defined as 1) development or worsening of regional wall motion abnormality, 2) reduction in left ventricular ejection fraction ≥ 8%, and/or 3) ischemic ST-segment changes during one or more of the three mental stress tests.
Results
A total of 98 (43.7%) out of 225 patients exhibited MSIMI. Patients developing MSIMI had significantly lower s′ (7.0±1.7 vs 7.5±1.2, p=0.016) and a′ (8.9±1.8 vs 10.0±1.9, p<0.001) at baseline whereas e′ did not differ (6.5±1.7 vs. 6.5±1.8, p=0.85). Furthermore, the eas-index was significantly higher (0.11±0.04 vs. 0.09±0.03, p<0.0001).The eas-index remained significantly associated with the propensity for developing MSIMI (Odds ratio per 0.05 unit increase: 1.85; 95%CI: 1.21–2.82, p=0.004) after adjustment of resting LVEF, resting wall motion index score, gender and social circumstances of living. There was no association between resting eas-index and ESIMI.
Conclusion
MSIMI but not ESIMI is independently associated with resting abnormalities in myocardial systolic and late diastolic velocities as well as the integrated measure of the eas-index in patients with known coronary artery disease.
Keywords: Mental stress induced ischemia, echocardiography, tissue Doppler
Background
Mental stress induced myocardial ischemia (MSIMI) is prevalent in patients with coronary artery disease1, 2 (CAD) and portends adverse outcome independently of traditional risk factors in this population3. Several studies have demonstrated distinct physiological alterations coupling mental stress to cardiovascular changes such as dynamic reductions in coronary blood flow demonstrated during mental stress in patients with CAD4, 5 and exaggerated catecholaminergic response during mental stress with increased systemic vascular resistance, opposite of the pattern seen during exercise6. Recently, treatment with Escitalopram has been demonstrated to reduce the rate of MSIMI in patients with CAD in the REMIT (Responses of Myocardial Ischemia to Escitalopram Treatment) trial7.
The available data suggests that MSIMI may be present in 45–60% of CAD patients usually co-existing with exercise induce ischemia (ESIMI); a smaller subset also exhibits isolated MSIMI6, 8. The propensity for developing MSIMI correlates with female gender, marital status and social living arrangements8. Tissue Doppler imaging (TDI) enables the assessment of myocardial annular velocities during systole (s′), and early as well as late diastole (e′ and a′ respectively) which confer important prognostic information in CAD9. A combination of these indices has been proposed as the eas-index (e′/(a′ x s′)) which combines the individual velocities while accounting for their significant interrelationship10. Both annular LV TDI velocities and the eas-index have been associated with adverse outcome in the general population10 but have never been studied in relation to MSMI. Abnormal myocardial velocities have been associated with hypertensive heart disease11 and abnormal ventricular-vascular coupling12 suggesting that the eas-index as an integrated measure of myocardial function and development of MSIMI could be related.
In this study we sought to assess the association between resting myocardial annular velocities and the propensity for developing MSIMI in a subgroup of patients screened for inclusion in the REMIT study (NCT00574847)13.
Methods
Patients with documented CAD, aged 21 years or older were recruited for screening in the REMIT study. Patients were eligible for the randomized REMIT study if they demonstrated MSIMI during the screening procedure. The study protocol was approved by the Duke University Health System Institutional Review Board. All participants provided written informed consent prior to screening. Detailed inclusion and exclusion criteria have previously been published13.
Study procedures
Detailed description of the study procedure has previously been published13. Briefly, participants were interviewed with the purpose of obtaining clinical characteristics, structured psychiatric assessment, psychometric tests and afterwards followed by measurements of resting vital parameters.
The details of the mental stress testing procedure have previously been published8, 13. Briefly, participants underwent 3 mental stress tasks in the following sequence with 6 min resting in between: 1) mental arithmetic (MS1); 2) mirror tracing (MS2); and 3) anger recall public speech (MS3). Finally, after a resting period patients underwent an exercise treadmill test (ES) using the Bruce protocol.
Assessment of myocardial ischemia
Echocardiography and ECG were used to assess myocardial ischemia. Blood pressure, heart rate, and standard 12-lead ECG were recorded simultaneously with the echocardiographic examination. Echocardiography was performed utilizing the Philips iE33 platform (Philips Ultrasound, Bothell, Washington). Left ventricular (LV) wall motion was graded qualitatively according to the American Society of Echocardiography’s recommended 16-segment model14. Each segment was graded as either normal, hypokinetic, akinetic, dyskinetic, or aneurismal; scores = 1,2,3,4, or 5, respectively. The sum of all segment scores divided by the number of scored segments constituted the wall motion score index (WMSI). Left ventricular ejection fraction (LVEF) was calculated according to the biplane Simpson method14. All analyses of LV wall motion were performed by two experienced cardiologists (E.J.V, Z.S.) with a kappa value for intra-/intervariability ranging from 0.80 to 0.87. Biplane LVEF was calculated by one experienced reader (B.S) and repeated in 15 random subjects by an experienced cardiologist (F.A.E) with no significant systematic difference (bias: −1.7 (p=0.6646)) and an intra-class coefficient of correlation of 0.90 (95% CI: 0.74–0.97).
Stress induced myocardial ischemia was defined by 1 or more of the following: 1) development of new or a worse wall motion abnormality; 2) reduction in LVEF ≥ 8%; and/or 3) horizontal or downsloping ST-segment depression ≥ 1 mm in at least 2 or more leads lasting ≥3 consecutive beats. MSIMI was defined by the aforementioned ischemic changes during 1 or more of the 3 mental stress tasks13.
Doppler echocardiography
Doppler recordings were performed at baseline and during each mental stress task (MS1, MS2 and MS3). Doppler recording of mitral inflow was performed by placing a 2.5 mm sample volume at the tip of the mitral valve (MV) leaflets during diastole. Peak velocity of early (E) and late (A) MV inflow were recorded as well as MV deceleration time (DT). Pulsed wave TDI traces were obtained from the lateral mitral annulus in the 4-chamber view using a 2.5 mm sample volume. From the average of three cardiac cycles we calculated peak systolic velocity (s′), early diastolic velocity (e′) and late diastolic velocity (a′). From the myocardial velocities, an integrated index (e′ / (a′ x s′)) termed the eas-index10 was calculated. All TDI measurements were performed by an experienced cardiologist (F.A.E) and repeated in 15 random subjects by another experienced reader (M.E) with excellent reproducibility for the eas-index (bias: −0.005 (p=0.7240); 95% limits of agreement: −0.024-0-015).
Statistical analysis
All data are reported as mean ± SD or median (first and third quartile, Q1–Q3). Baseline clinical and echocardiographic characteristics are given according to tertiles of the eas-index and whether patients developed MSIMI. Student t test and Chi2 test were used for continuous and categorical variables respectively and all tests were two-sided with a significance level set at p< 0.05. Correlation analyses between continuous variables were performed with the Spearman correlation coefficient. The association between resting echocardiographic parameters, clinical information and MSIMI was analyzed with multiple logistic regression analysis with the binary outcome MSIMI as previously defined. The association between resting eas-index and MSIMI was adjusted for resting LVEF, resting WMSI and variables combining information on gender, marital status and living arrangements which have previously been associated with MSIMI8. Finally, we compared the resting eas-index between patients with isolated MSIMI versus isolated ESIMI. All statistical analyses were performed using SAS version 9.2 (SAS; Cary, North Carolina) and R: A language and environment for statistical computing (Version 2.15.2; Vienna, Austria).
Results
Baseline characteristics
Out of 335 patients who presented for baseline mental stress testing; a total of 225 patients had complete data on all stress tests and TDI measurements at baseline. Reasons for not fulfilling requirements for the present study were as follows: Poor acoustic window (n=19); cancellation due to safety concern (n=6); Un-interpretable mental stress test images or Uninterpretable exercise stress test images (n=20); and missing TDI recordings (n=65).
The baseline characteristics according to eas-index tertiles and MSIMI status are given in Table 1 and 2 respectively. Patients with higher eas-index were more likely to be younger (p=0.0297), female (p=0.0267) and un-married (p=0.0060). However, there was no difference in resting LVEF (p=0.5819), WMSI (p=0.1279) or E/e′ ratio (p=0.0793). Overall, results of the exercise- and mental stress tests were as follows: 105 patients (46.7%) developed neither MSIMI nor ESIMI; 22 (9.8%) developed isolated ESIMI; 53 (23.6%) developed both MSIMI and ESIMI; and 45 (20.0%) developed isolated MSIMI. Thus, a total of 98 (43.5%) developed MSIMI either isolated or in combination with ESIMI. Among the 98 patients who developed MSIMI, 60 (61.2%) developed only new or worsening wall motion abnormalities, 19 (19.4%) developed only a drop in LVEF ≥ 8% and 19 (19.4%) had a combination of wall motion abnormalities and drop in LVEF ≥ 8%, during one or more mental stress tasks. No patients had ST segment changes during the MS tasks. Among the 98 patients with MSIMI, ischemia was detected in 51 (52.0%) during mental arithmetic; 66 (67.3%) during mirror tracing and 73 (74.5%) during anger recall speech.
Table 1.
Baseline and echocardiographic characteristics according to tertiles of eas-index
| Characteristic | Eas <0.08 n = 75 |
0.08< eas <0.11 n = 75 |
eas >0.11 n = 75 |
p-value |
|---|---|---|---|---|
| Age | 65.6 ±8.2 | 62.7 ±10.2 | 61.4 ±11.1 | 0.0297 |
| Race, white | 66 (88.0) | 61 (81.3) | 62 (82.7) | 0.4994 |
| Female gender | 7 (9.3) | 11 (14.7) | 19 (25.3) | 0.0267 |
| BMI, kg/m2 | 28.68 ±4.35 | 29.27 ±4.47 | 28.19 ±4.60 | 0.3534 |
| Living arrangements, alone | 8 (10.7) | 12 (16.0) | 14 (18.7) | 0.3790 |
| Marital status, not married | 11 (14.7) | 13 (17.3) | 26 (34.7) | 0.0060 |
| Current smoker | 3 (4.0) | 11 (14.7) | 14 (18.7) | 0.0642 |
| Current medical therapy | ||||
| ASA | 73 (97.3) | 70 (94.6) | 72 (97.3) | 0.5891 |
| Other antiplatelet agent | 34 (45.3) | 36 (48.0) | 26 (35.1) | 0.2466 |
| ACEi | 48 (64.0) | 46 (61.3) | 47 (63.5) | 0.9373 |
| ARB | 11 (14.9) | 16 (21.3) | 5 (6.8) | 0.0395 |
| Beta-blocker | 62 (82.7) | 58 (77.3) | 65 (87.8) | 0.2394 |
| Calcium channel blocker | 19 (25.3) | 18 (24.3) | 12 (16.2) | 0.3391 |
| Statin | 69 (93.2) | 66 (89.2) | 70 (94.6) | 0.4369 |
| History of MI | 39 (52.0) | 31 (41.3) | 28 (37.3) | 0.3740 |
| History of CABG | 29 (38.7) | 29 (38.7) | 36 (48.0) | 0.4085 |
| History of PCI | 47 (62.7) | 49 (65.3) | 47 (62.7) | 0.9261 |
| History of DM | 22 (29.3) | 23 (30.7) | 18 (24.0) | 0.6294 |
| History of HTN | 60 (80.0) | 58 (77.3) | 58 (77.3) | 0.9009 |
| History of hyperlipidemia | 70 (93.3) | 71 (94.7) | 69 (92.0) | 0.8071 |
| NYHA functional class | ||||
| I | 68 (90.7) | 70 (93.3) | 67 (89.3) | |
| II | 6 (8.0) | 4 (5.3) | 5 (6.7) | 0.4268 |
| III | 1 (1.3) | 1 (1.3) | 3 (4.0) | |
| History of depression | 8 (10.7) | 9 (12.0) | 11 (14.7) | 0.7516 |
| Resting LVEF, % | 58.5 ±9.3 | 59.0±8.4 | 57.4 ±9.8 | 0.5819 |
| Resting WMSI | 1.16 ±0.32 | 1.09 ±0.20 | 1.19 ±0.37 | 0.1279 |
| Resting MV dec time, ms | 245.3 ±63.2 | 225.2 ±50.7 | 220.0 ±59.9 | 0.0219 |
| Resting E/A ratio | 0.85 ±0.24 | 1.02 ±0.28 | 1.07 ±0.38 | < |
| Resting E/e′ | 12.8 ±4.2 | 11.8 ±4.3 | 11.2 ±4.6 | 0.0793 |
| Resting eas-index | 0.07 ±0.01 | 0.09 ±0.01 | 0.14 ±0.03 | < |
| MSIMI | 23 (30.7%) | 30 (40.0) | 45 (60.0) | 0.0011 |
Table 2.
Baseline and echocardiographic characteristics according to presence of MSIMI
| Characteristic | No MSIMI N=127 |
MSIMI N=98 |
p-value |
|---|---|---|---|
| Age | 63.5 ±10.0 | 62.8 ±10.0 | 0.5861 |
| Race, white | 109 (85.8) | 80 (81.6) | 0.5045 |
| Female gender | 17 (13.4) | 20 (20.4) | 0.2196 |
| BMI, kg/m2 | 29.0 ±4.5 | 28.4 ±4.5 | 0.3256 |
| Living arrangements, alone | 15 (11.8) | 19 (19.4) | 0.1658 |
| Marital status, not married | 23 (18.1) | 27 (27.6) | 0.1267 |
| Current smoker | 12 (9.4) | 16 (16.3) | 0.2302 |
| Current medical therapy | |||
| ASA | 121 (96.0) | 94 (96.9) | 1.0000 |
| Other antiplatelet agent | 50 (39.4) | 46 (47.4) | 0.2844 |
| ACEi | 79 (62.2) | 62 (63.9) | 0.9018 |
| ARB | 15 (11.9) | 17 (17.5) | 0.3201 |
| Beta-blocker | 103 (81.1) | 82 (84.5) | 0.6215 |
| Calcium channel blocker | 29 (23.0) | 20 (20.6) | 0.7906 |
| Statin | 113 (89.7) | 92 (95.8) | 0.1463 |
| History of MI | 51 (40.2) | 47 (48.0) | 0.4844 |
| History of CABG | 51 (40.2) | 43 (43.9) | 0.6711 |
| History of PCI | 79 (62.2) | 64 (65.3) | 0.7342 |
| History of DM | 38 (29.9) | 25 (25.5) | 0.7342 |
| History of HTN | 102 (80.3) | 74 (75.5) | 0.4821 |
| History of hyperlipidemia | 119 (93.7) | 91 (92.9) | 1.0000 |
| NYHA functional class | |||
| I | 115 (90.6) | 90 (91.8) | |
| II | 8 (6.3) | 7 (7.1) | 0.7053 |
| III | 4 (4.1) | 1 (1.0) | |
| History of depression | 15 (11.8) | 13 (13.3) | 0.9013 |
| Resting LVEF, % | 58.7 ±8.2 | 57.8 ±10.3 | 0.4817 |
| Resting WMSI | 1.11 ±0.28 | 1.19 ±0.34 | 0.0725 |
| Resting MV dec time, ms | 228.1 ±58.2 | 232.6 ±60.0 | 0.5726 |
| Resting E/A ratio | 0.97 ±0.34 | 1.00 ±0.30 | 0.4976 |
| Resting E/e′ | 11.7 ±4.2 | 12.2 ±4.7 | 0.4785 |
| Resting eas-index | 0.09 ±0.03 | 0.11 ±0.04 | < 0.0001 |
Baseline s′ correlated significantly with both e′ (rho=0.42, p<0.0001) and a′ (rho=0.49, p<0.0001) whereas the correlation between e′ and a′ was less strong (rho=0.24, p<0.0001).
Myocardial velocities in relation to MSIMI
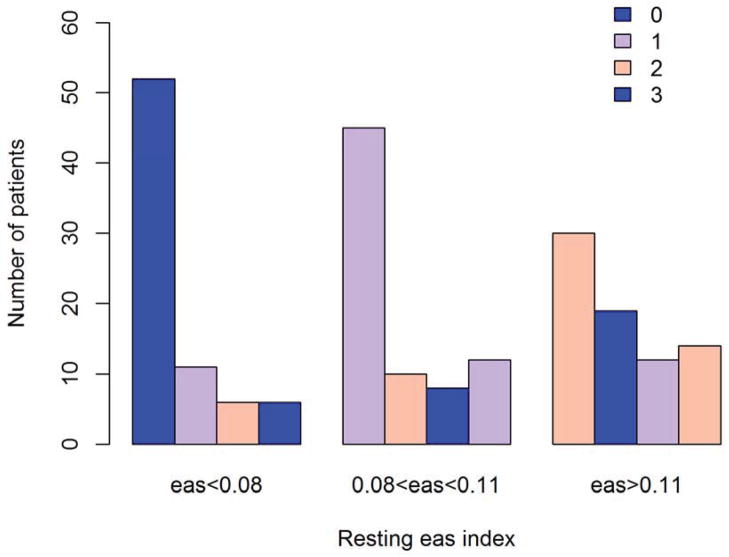
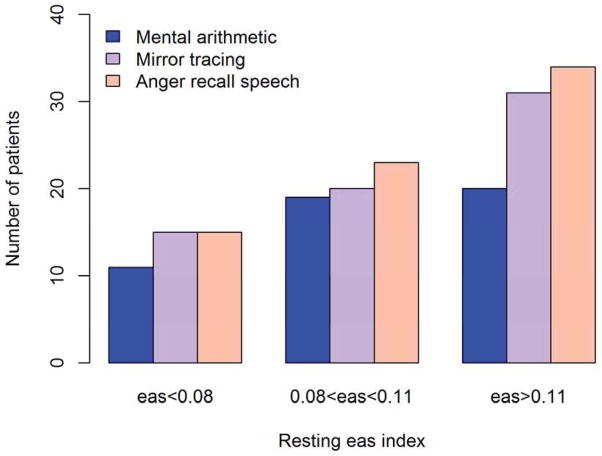
Patients developing MSIMI were more likely to be living alone (19.4% vs 11.8%), be unmarried (27.6% vs 18.1%) and be female (20.4% vs. 13.4%), however, none of these differences were statistically different in the present subset of the REMIT-screening patients (all p>0.05). Development of MSIMI was associated with higher resting WMSI (1.19±0.34 vs 1.11±0.28, p=0.0725) whereas resting LVEF did not differ significantly (57.8±10.3 vs 58.7± 8.2, p=0.4817). The eas-index was significantly higher in patients developing MSIMI compared to those without MSIMI (0.11± 0.04 vs 0.09± 0.03, p<0.0001). The cumulative number of positive MS tasks was significantly higher among patients in the highest tertile of the eas-index (p=0.005) (figure 2). The number of patients having MSIMI by each MS task was proportionally higher with increasing tertile of the eas-index (Figure 3) however, only mirror tracing (MS2) (p=0.0137) and anger recall speech (MS3) (p=0.0039) were statistically significant, whereas mental arithmetic was not significant (p=0.1543).
Figure 2. Positive mental stress tasks according to resting eas-index.
Cumulative number of positive mental stress tasks according to tertiles of resting eas-index.
Figure 3. Positive response to mental stress tasks according to resting eas-index.
Distribution of mental stress induced ischemia by mental stress task according to tertiles of resting eas-index.
Among the individual components of the eas-index only resting s′ (7.0 ±1.7 vs 7.5±1.2, p=0.0205) and a′ (8.9 ±1.8 vs 10.0 ±1.9, p<0.0001) differed between patients exhibiting MSIMI, whereas e′ (6.5 ±1.7 vs 6.5 ±1.8, p=0.8475) did not.
There was a significant correlation between mean change in LVEF from baseline to all 3 mental stress tests and resting LVEF (rho=−0.30, p<0.0001), resting s′ (rho=0.20, p=0.0034), resting a′ (rho=0.17, p=0.0132), MV dec time (rho=−0.15, p=0.0311) and eas-index (rho=−0.17, p=0.0106). There was no correlation between resting e′ (rho=0.06, p=0.4195) and resting WMSI (rho=−0.01, p=0.8341). Patients with female gender (−1.18± 4.61 vs −0.31±4.75, p=0.3234), non-married status (−1.40± 6.20 vs −0.18±4.17, p=0.2024) and single living (−2.11± 6.72 vs −0.17± 4.24, p=0.1275) had more pronounced changes in LVEF during mental stress testing however, none of these differences were statistically significant.
Multivariable logistic regression analysis revealed that eas-index was independently related to MSIMI after adjustment for age, resting LVEF, resting WMSI, female gender, single living and un-married status (OR per 0.05 unit increase: 1.85; 95%CI: 1.21–2.82, p=0.0043). In the adjusted model neither resting LVEF (OR: 1.01; 95%CI: 0.97–1.05, p=0.7376), resting WMSI (OR per 0.1 unit increase: 1.09; 95%CI: 0.96–1.22, p=0.1780), age (OR: 1.01; 95%CI: 0.98–1.04, p=0.5232), female gender (OR: 1.01; 95%CI: 0.43–2.39, p=0.9769), single living (OR: 1.38; 95%CI: 0.42–4.55, p=0.5931) nor un-married status (OR: 1.10; 95%CI: 0.37–3.27, p=0.8661) maintained significant association with the development of MSIMI. When adjusting for a combination of gender and single living, higher eas-index was borderline significantly associated with MSIMI (OR per 0.05 unit increase: 1.34; 95%CI: 0.92–1.94, p=0.1235) whereas a non-significant association was found for single female vs non-single male (OR: 1.83: 95%CI: 0.62–5.39, p=0.5492) and single male vs non-single male (OR: 1.97; 95%CI: 0.75–5.16, p=0.4055).
Patients with isolated MSIMI had higher eas-index compared to patients with isolated ESIMI (0.11 ±0.04 vs 0.09 ±0.03, p=0.0224). After adjusting for the combination of gender and single living as above, eas-index remained independently associated with isolated MSIMI (OR per 0.05 unit increase: 2.50; 95%CI: 1.02–6.17, p=0.0462).
Changes in myocardial velocities during mental stress
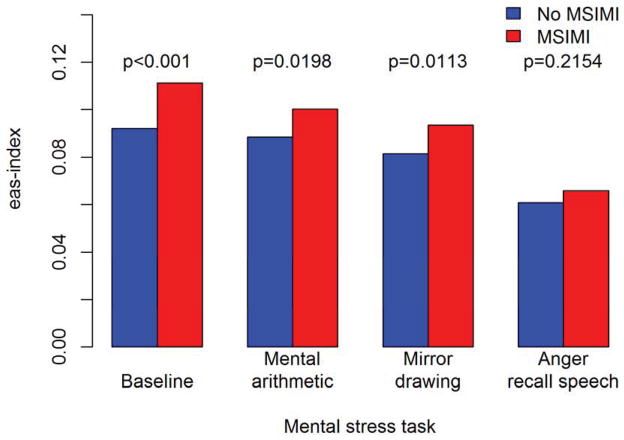
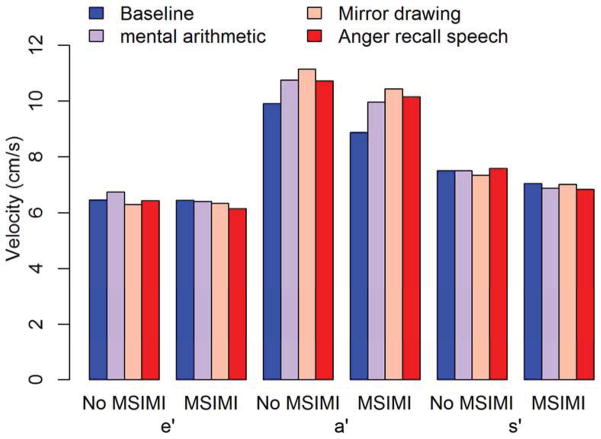
Between group (MSIMI vs. no MSIMI) differences in the eas-index remained significant during the mental arithmetic (p=0.0198) and mirror drawing (p=0.0133) tasks, whereas no significant difference was found during the anger recall speech task (p=0.2154). Decreasing eas-index was seen for patients with and without MSIMI across the three MS tasks (Figure 4). The decrease in the eas-index was driven by increases in a′, whereas e′ and s′ remained constant although the patients developing MSIMI continued to exhibit relatively lower a′ and s′ velocities (Figure 5).
Figure 4. eas-index during mental stress tests.
Relationship between the eas-index obtained at each mental stress task according to whether patients developed MSIMI.
Figure 5. Pattern of annular myocardial velocities during mental stress.
Relationship between myocardial annular velocities (e′, a′ and s′) obtained at each mental stress task according to whether the patient developed MSIMI.
Discussion
The main findings of the present study can be summarized as follows: 1) In a population with stable CAD we demonstrate that an integrated measure of annular myocardial velocities is associated with the propensity for developing MSIMI; 2) an integrated measure of myocardial velocities correlate with demographic characteristics which have been linked to MSIMI in previous studies8.
Mechanisms underlying MSIMI
The underlying mechanisms of MSIMI are not fully understood however, several distinctive features have been identified that set MSIMI apart from ESIMI. The myocardial ischemic response to mental stress occurs at a lower rate pressure product compared to ESIMI implying that MSIMI is present at lower levels of oxygen demand6. Although the blood pressure rise accompanying MSIMI is relatively smaller in magnitude compared to that seen with ESIMI, a distinctive feature of MSIMI seems to be the increase in systemic vascular resistance which is opposite of ESIMI6. The mechanism for this response is not elucidated however, it has been suggested that central neurogenic peripheral vasoconstriction may be involved1. Studies utilizing functional magnetic resonance imaging have demonstrated distinct areas in the medial prefrontal cortex that together modulate cardiovascular response to mental stress with significant individual differences15. Abnormal myocardial systolic velocities have also been demonstrated to be independently associated with increased vascular stiffness and abnormal ventricular-vascular coupling11, 12. We cannot however from the present data draw any definite conclusions on the association between myocardial tissue velocities and peripheral vasoconstriction at baseline or during mental stress in patients exhibiting MSIMI.
Abnormal coronary vasomotor response has been implicated in the pathogenesis of MSIMI. Compared to healthy controls, patients with CAD did not demonstrate increased coronary blood flow during mental stress and local inability of endothelium mediated vasodilation in diseased coronary vessels has been implicated in the occurrence of MSIMI5. Other studies have demonstrated blunted myocardial blood flow in normal epicardial arteries in response to mental stress suggestive of microvascular dysfunction16. Subclinical myocardial dysfunction assessed by TDI has been associated with abnormal coronary flow reserve in patients with hypertension, diabetes and recent acute myocardial infarction17–19. This could suggest that lower resting myocardial velocities in patients with a propensity for developing MSIMI may reflect pre-existing microvascular dysfunction.
MSIMI in relation to annular myocardial velocities
The observed independent association between resting eas-index and MSIMI could reflect a shared etiology with both of these being affected by a complex interplay of neurogenic vasoactive response, inhomogeneous coronary vasoreactivity and myocardial microvascular dysfunction. The present study cannot give any indication of causality between impaired resting myocardial velocities and MSIMI. However, to the best of our knowledge this is the first study to demonstrate resting myocardial abnormalities in patients exhibiting MSIMI. Interestingly, factors previously associated with increased propensity for MSIMI, namely female gender and marital status8 were also associated with increased eas-index in this study.
The rationale for integrating myocardial annular velocities has previously been proposed10. The significant internal dependency of annular myocardial velocities was confirmed in our study where s′ correlated significantly with both a′ and e′ whereas the correlation between e′ and a′ was less pronounced. Decreases in e′ are associated with normal aging due to the progressive age-related impairment of myocardial active relaxation20 whereas a′ increases. However, progressive myocardial passive stiffness due to loss of visco-elastic properties will lead to additional reductions in a′ which heralds adverse outcome especially when accompanied by low s′ 21. Low s′ is seen with deteriorating LV long axis systolic function and has been demonstrated to improve the diagnostic accuracy of suspected CAD independently of exercise ECG and conventional echocardiographic parameters22. Furthermore, impaired long axis LV systolic function leads to less potential energy being stored during systole which directly interacts with subsequent early diastole through diminished elastic recoil23. The integrated approach towards the myocardial annular velocities was favored by Mogelvang et al. due to the observed close relationship between aging and e′ in the normal population10. The REMIT study included a higher risk population of patients with known CAD which is different from the population described by Mogelvang et al. However, the association between the eas-index and MSMI reported in our study does not impact the documented relationship between e′ and long term clinical outcome in patients with CAD9. Taken together, these considerations suggest that an integrated approach to quantifying myocardial velocities should be more appropriate than assessing each parameter in isolation10, 21.
During the MS tasks the eas-index decreased in similar fashion whether or not the patients developed MSIMI and this change was driven by relatively unchanged s′ and e′ velocities whereas a′ increased. Higher a′ values are seen in patients with impaired relaxation compared to the more advanced pseudo normal pattern and key determinants of a′ have previously been identified as LA dP/dt, LA relaxation and LVEDP24–26. Whether MS tasks induce changes in LA function cannot be conclusively answered from the present study due to lack of invasive measurements and high resolution LA planimetry. These findings deserve further exploration in future studies of MSIMI.
Limitations
Several limitations must be noted for this study. First and foremost TDI assessment was not available in all patients completing the REMIT screening program which diminishes our sample size compared to prior results8. Recordings of TDI were only available for the lateral annulus of the 4-chamber view which limits our ability to more accurately assess the global myocardial velocities. More accurate measures of global LV function such as strain27 could potentially have added further information with regard to development of MSIMI however; these novel parameters were not assessed in the REMIT screening program13.
Conclusion
An integrated measure of resting myocardial velocities is independently associated with the propensity for developing MSIMI in patients with CAD. These novel results imply that sub clinical myocardial dysfunction may co-exist with previously described pathophysiological alterations in peripheral vasculature and coronary vasoreactivity in patients with MSIMI.
Figure 1. Example of different annular myocardial velocity pattern in patients with- and without MSIMI.
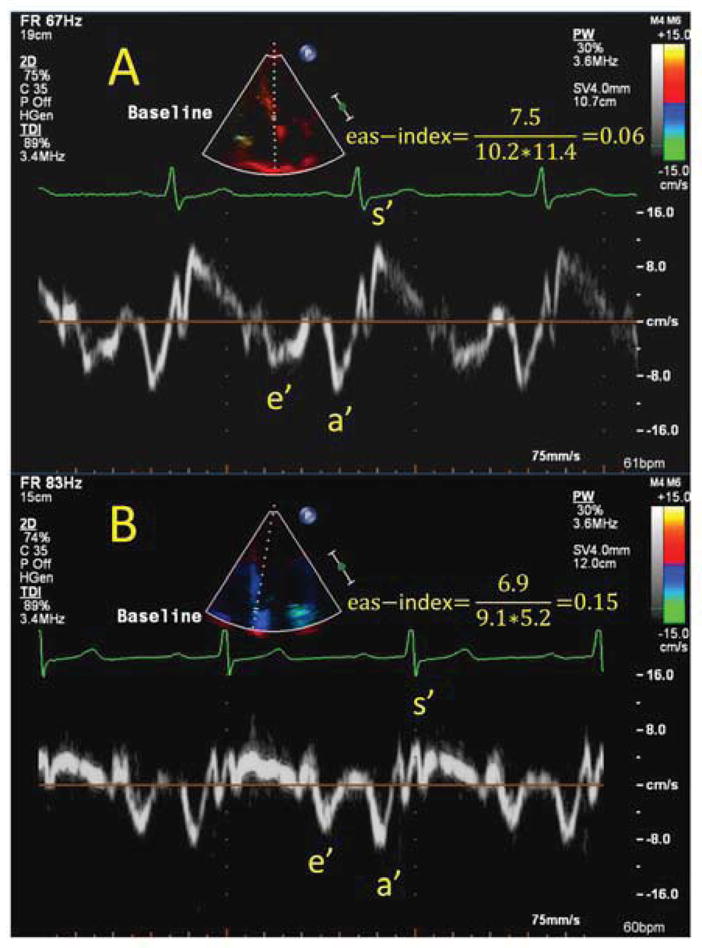
Example of two patients exhibiting differential patterns of response towards mental stress- and exercise stress test. Patient A (Top) had preserved myocardial velocities at baseline with an eas-index (e′/(a′x s′)) of 0.06 and had a positive exercise stress test but negative mental stress test (isolated ESIMI). Patient B (bottom) had impaired baseline myocardial velocities with a markedly reduced s′ and an eas-index of 0.15 and experienced isolated MSIMI. Both patient A and patient B had preserved LVEF (61% and 57% respectively).
Acknowledgments
The REMIT study was funded by the National Heart, Lung, and Blood Institute (NHLBI, R01HL085704), Bethesda, Maryland.
Abbreviations
- MSIMI
Mental stress induced ischemia
- ESIMI
Exercise stress induced ischemia
- TDI
Tissue Doppler imaging
- LVEF
left ventricular ejection fraction
- CAD
Coronary artery disease
- WMSI
Wall motion scoring index
Footnotes
Disclosures: The authors report the following potential conflicts of interest: Dr. Samad is a sub-investigator for the REALISM (Everest II Real World ExpAnded MuLtIcenter Study of the MitraClip System) funded by Abbott Vascular. Dr. O’Connor is a co-owner of Biscardia, a stockholder in Neurotronik/Interventional Autonomics Corporation (Stockholder), and has received financial support from Actelion Pharmaceuticals, Amgen, Astellas Pharma, BG Medicine, Critical Diagnostics, GE Healthcare, Gilead Sciences, HeartWare, Ikaria, Johnson & Johnson, Novartis, Otsuka Pharmaceutical Company, Pfizer, Pozen, ResMed, and Roche Diagnostics. Dr. Velazquez is a consultant for Novartis, and has received research grants from Abbott Vascular and Ikaria Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. European heart journal. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O’Connor CM, Blumenthal JA. Mental stress--induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 4.Legault SE, Freeman MR, Langer A, Armstrong PW. Pathophysiology and time course of silent myocardial ischaemia during mental stress: Clinical, anatomical, and physiological correlates. British heart journal. 1995;73:242–249. doi: 10.1136/hrt.73.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M, Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: Relationship with hemodynamic stress responses. Journal of the American College of Cardiology. 2001;37:1359–1366. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the psychophysiological investigations of myocardial ischemia study (pimi) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 7.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. Journal of the American College of Cardiology. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers J, Kuchibhatla M, O’Connor C, Velazquez EJ. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. Journal of the American College of Cardiology. 2013;61:714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue doppler imaging a new prognosticator for cardiovascular diseases. Journal of the American College of Cardiology. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 11.Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. Journal of the American College of Cardiology. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Velazquez EJ, Samad Z, Kuchibhatla M, Martsberger C, Rogers J, Williams R, Kuhn C, Ortel TL, Becker RC, Pristera N, Krishnan R, O’Connor CM. Responses of mental stress-induced myocardial ischemia to escitalopram treatment: Background, design, and method for the responses of mental stress induced myocardial ischemia to escitalopram treatment trial. American heart journal. 2012;163:20–26. doi: 10.1016/j.ahj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf Fa, Foster E, Pellikka Pa, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MSJ, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiograph. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: Part i: Reciprocal dorsal and ventral subregions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL, Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–311. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 17.D’Andrea A, Nistri S, Castaldo F, Galderisi M, Mele D, Agricola E, Losi MA, Mondillo S, Marino PN. The relationship between early left ventricular myocardial alterations and reduced coronary flow reserve in non-insulin-dependent diabetic patients with microvascular angina. International journal of cardiology. 2012;154:250–255. doi: 10.1016/j.ijcard.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Logstrup BB, Hofsten DE, Christophersen TB, Moller JE, Botker HE, Pellikka PA, Egstrup K. Correlation between left ventricular global and regional longitudinal systolic strain and impaired microcirculation in patients with acute myocardial infarction. Echocardiography. 2012 doi: 10.1111/j.1540-8175.2012.01784.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidis I, Tzortzis S, Paraskevaidis I, Triantafyllidi H, Papadopoulos C, Papadakis I, Trivilou P, Parissis J, Anastasiou-Nana M, Lekakis J. Association of abnormal coronary microcirculatory function with impaired response of longitudinal left ventricular function during adenosine stress echocardiography in untreated hypertensive patients. European heart journal cardiovascular Imaging. 2012;13:1030–1040. doi: 10.1093/ehjci/jes071. [DOI] [PubMed] [Google Scholar]
- 20.Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of left ventricular diastolic function with two-dimensional and doppler echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1997;10:246–270. doi: 10.1016/s0894-7317(97)70062-2. [DOI] [PubMed] [Google Scholar]
- 21.Andersson C, Gislason GH, Mogelvang R, Hoffmann S, Merie C, Kober L, Torp-Pedersen C, Sogaard P. Importance and inter-relationship of tissue doppler variables for predicting adverse outcomes in high-risk patients: An analysis of 388 diabetic patients referred for coronary angiography. European heart journal cardiovascular Imaging. 2012;13:643–649. doi: 10.1093/ejechocard/jer297. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann S, Jensen JS, Iversen AZ, Sogaard P, Galatius S, Olsen NT, Bech J, Fritz-Hansen T, Biering-Sorensen T, Badskjaer J, Pietersen A, Mogelvang R. Tissue doppler echocardiography improves the diagnosis of coronary artery stenosis in stable angina pectoris. European heart journal cardiovascular Imaging. 2012;13:724–729. doi: 10.1093/ehjci/jes001. [DOI] [PubMed] [Google Scholar]
- 23.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue doppler. Journal of the American College of Cardiology. 2001;37:278–285. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. Journal of the American College of Cardiology. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez L, Garcia M, Ares M, Griffin BP, Nakatani S, Thomas JD. Assessment of mitral annular dynamics during diastole by doppler tissue imaging: Comparison with mitral doppler inflow in subjects without heart disease and in patients with left ventricular hypertrophy. American heart journal. 1996;131:982–987. doi: 10.1016/s0002-8703(96)90183-0. [DOI] [PubMed] [Google Scholar]
- 27.Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, Kober L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]