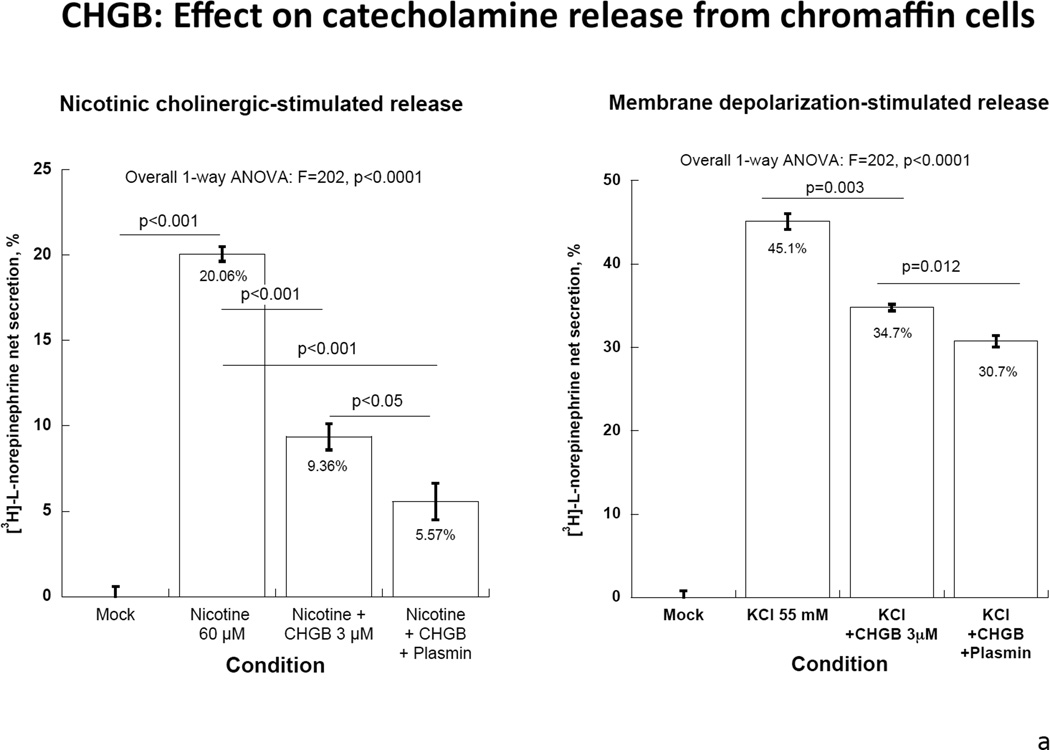
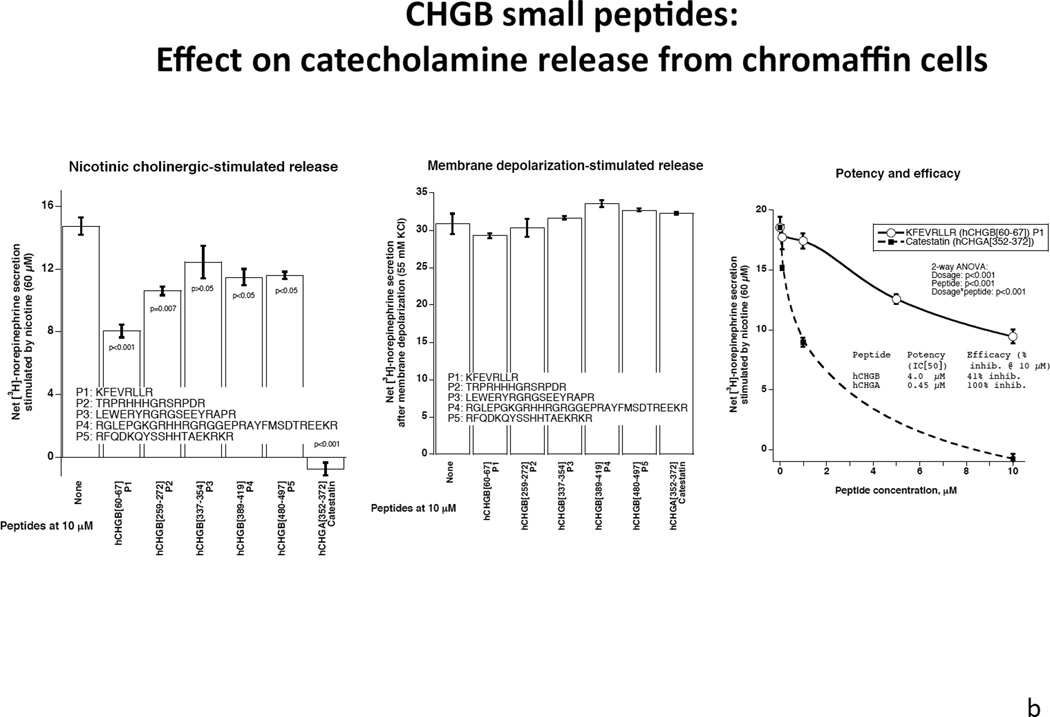
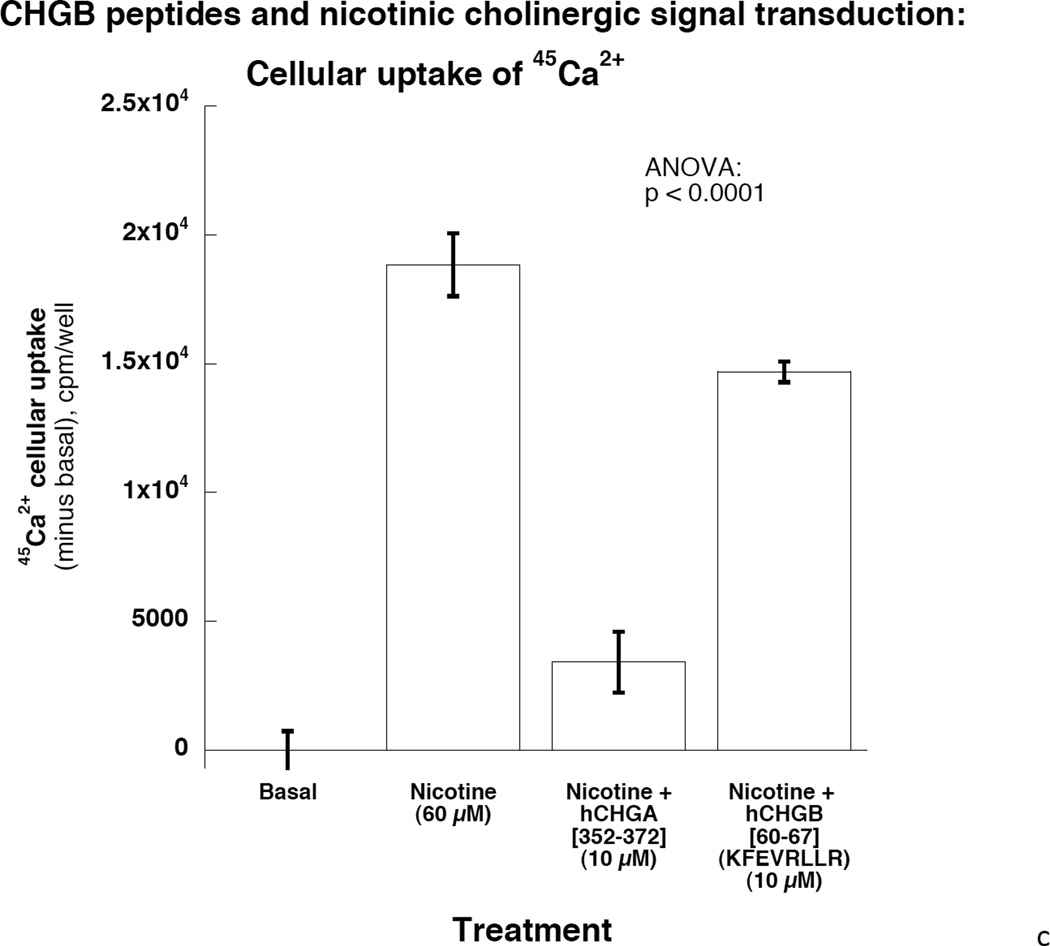
Figure 4. Extracellular CHGB effects on catecholamine release.
Each experiment was repeated 3 times.
4a: Human CHGB protein or its proteolytically-digested fragments on nicotinic cholinergic versus membrane depolarization (KCl) -stimulated catecholamine release. CHGB protein or its plasmin-digested fragments inhibited predominantly nicotine-stimulated catecholamine release. 4b: Left: Synthetic CHGB peptides on nicotinic cholinergic stimulated catecholamine release. Catestatin (human CHGA[352–372]) served as a positive inhibitory control. Peptide human CHGB[60–67] displayed the greatest inhibitory effect among these peptides. P value is displayed for each peptide compared with negative control. Middle: Synthesized Human CHGB peptides on membrane depolarization (KCl)- stimulated catecholamine release. Catestatin (human CHGA[352–372]) served as a positive inhibitory control. No inhibitory effect was observed for these peptides (p>0.05 for each peptide).
Right: Potency and efficacy of CHGB[60–67] on nicotinic cholinergic-stimulated catechalmine release. Catestatin (human CHGA[352–372]) served as a positive control. Potency and efficacy of CHGB[60–67] are displayed. 4c: CHGB peptide effect on nicotinic cholinergic cationic (Ca2+) signal transduction in chromaffin cells. PC12 cells were treated with 45Ca2+ at 2 µCi/ml and incubated under different conditions including basal, nicotine alone at 60 µM, and nicotine with hCHGA or hCHGB peptides. Catestatin (human CHGA[352–372]) served as a positive control inhibitor. Human CHGB[60–67] moderately (by ~22%) inhibited nicotine-triggered uptake of 45Ca.