Abstract
Background
Variable airflow obstruction and airway hyperresponsiveness (AHR) are features of asthma, which are absent in nonasthmatic eosinophilic bronchitis (EB). Airway remodelling is characteristic of both conditions suggesting that remodelling and airway dysfunction are disassociated, but whether the airway geometry differs between asthma and nonasthmatic EB is uncertain.
Methods
We assessed airway geometry by computed tomography (CT) imaging in asthma vs EB. A total of 12 subjects with mild–moderate asthma, 14 subjects with refractory asthma, 10 subjects with EB and 11 healthy volunteers were recruited. Subjects had a narrow collimation (0.75 mm) CT scan from the aortic arch to the carina to capture the right upper lobe apical segmental bronchus (RB1). In subjects with asthma and EB, CT scans were performed before and after a 2-week course of oral prednisolone (0.5 mg/kg).
Results
Mild–moderate and refractory asthma were associated with RB1 wall thickening in contrast to subjects with nonasthmatic EB who had maintained RB1 patency without wall thickening [mean (SD) % wall area and luminal area mild-t0-moderate asthma 67.7 (7.3)% and 6.6 (2.8) mm2/m2, refractory asthma 67.3 (5.6)% and 6.7 (3.4) mm2/m2, healthy control group 59.7 (6.3)% and 8.7 (3.8) mm2/m2, EB 61.4 (7.8)% and 11.1 (4.6) mm2/m2 respectively; P < 0.05]. Airway wall thickening of non-RB1 airways generation three to six was a feature of asthma only. There was no change in airway geometry of RB1 after prednisolone. Proximal airway wall thickening was associated with AHR in asthma (r = −0.56; P = 0.02).
Conclusions
Maintained airway patency in EB may protect against the development of AHR, whereas airway wall thickening may promote AHR in asthma.
Keywords: airway hyperresponsiveness, asthma, computed tomography, eosinophilic bronchitis, geometry
Asthma is characterized by typical symptoms, variable airflow obstruction and airway hyperresponsiveness (AHR) in association with airway inflammation and structural changes within the airway wall, collectively known as remodelling (1). In bronchial biopsies from asthmatics, remodelling of different components of the airway wall including the vasculature, smooth muscle, glands, matrix; and the influx of CD8+ T-cells correlate with airflow obstruction (2, 3) and forced expiratory volume in 1 s (FEV1) decline (4). In contrast, the association between AHR and remodelling is unclear.
Nonasthmatic eosinophilic bronchitis (EB) is a powerful disease control model to study the potential mechanisms of AHR. It accounts for approximately 10% of the referrals to a specialist cough clinic (5) and is characterized by eosinophilic airway inflammation demonstrated by sputum, bronchoalveolar lavage and bronchial biopsy, without any evidence of variable airflow obstruction or AHR (6–9). We have demonstrated that vascular remodelling (2), thickening of the reticular basement membrane (7, 9), matrix deposition and increased airway smooth muscle mass (10) occur to a similar degree in asthma and EB, and it is therefore unlikely that these components of the remodelling process contribute to AHR in asthma. In spite of the evidence in favour of remodelling in EB, Park et al. (11) have recently reported that airway wall thickening of proximal airways was not a feature of EB, but confidence in this conclusion is undermined by the lack of standardization of airway measurements for subject size and the short duration of disease of 5–8 months (12), questioning whether these subjects had sufficient time to develop remodelling. Therefore, whether airway wall thickening is a feature of EB remains uncertain.
We hypothesized that remodelling is a feature of EB and asthma and that AHR and remodelling are independent. To test our hypothesis, we studied subjects with EB, mild-to-severe refractory asthma and healthy controls. We chose to use a limited narrow collimation computed tomography (CT) from the aortic arch to the carina to capture the right upper lobe apical segmental bronchus (RB1) a third generation conducting airway and non-RB1 airways (generations three to six), as the RB1 bronchus is a useful marker of remodelling in asthma (13). Computed tomography measures were extensively validated using a phantom and ex vivo sheep model with ‘gold’ standard measurements made by stereomicroscopy and micro-CT.
Methods
Subjects
In all, 12 subjects with mild-to-moderate asthma (GINA treatment steps 1–4) (14), 14 subjects with severe asthma [fulfilling the ATS refractory asthma definition (15) and GINA treatment step 5], 10 subjects with EB (8) and 11 healthy volunteers were prospectively recruited from Glenfield Hospital outpatients, staff and by local advertising. One of the patients with mild-to-moderate asthma withdrew after the baseline CT scan for nonmedical reasons. All subjects were nonsmokers with a smoking history of <10 pack years; had been free of exacerbations and on stable treatment for 8-week prior to entry into the study. The Leicestershire ethics committee approved the study and all subjects gave their written informed consent.
Protocol and clinical characterization
All subjects underwent clinical characterization including spirometry, allergen skin prick tests for common aeroallergens, measurement of exhaled nitric oxide (eNO) concentration (16), a methacholine inhalation test (17), sputum induction (18) and peripheral blood eosinophil count and total IgE.
Subjects had a limited CT scan from the aortic arch to the carina to capture the RB1. Computed tomography scanning was performed with a Siemens Sensation 16 multislice scanner (Siemens, Camberley, UK). Scans were obtained at 0.75 mm collimation, 120 kV, 50 mAs, pitch 1.1, scan length 53 mm and scan time of 2.85 s. Images were reconstructed at 0.75 mm slice thickness using a 512 × 512 matrix and a very sharp reconstruction algorithm (B70-f). Computed tomography scans were performed at full inspiration and patients were instructed to complete an adequate breath hold prior to scanning.
Subjects with mild-to-moderate asthma or EB had a limited CT before and after 2 weeks of treatment with prednisolone (0.5 mg/kg once daily), to quantify the effect of short-term prednisolone and to minimize the possible confounding effects of airway inflammation upon airway geometry. Severe refractory asthmatics underwent a single CT after treatment with prednisolone 0.5 mg/kg once daily for 2 weeks, because they were already on maintenance oral corticosteroids, thus limiting the validity of a preprednisolone trial CT scan. Healthy controls underwent a single CT. Computed tomography scanning was performed within 1 day of the prednisolone trial, and clinical characterization was performed within 5 days of the prednisolone trial.
Validation of airway geometry measurements
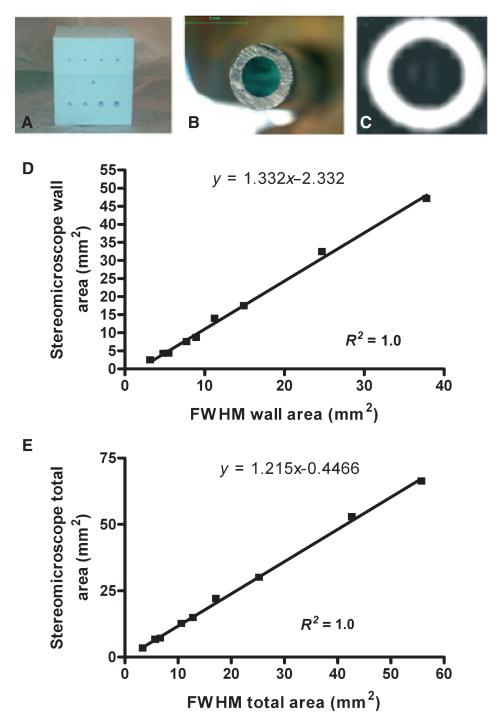
A phantom airway model (Fig. 1A) and ex vivo sheep airway model were used to validate the airway geometry measurements. The airway phantom was constructed from a polystyrene block with nine circular plastic tubes, dimensions [luminal area (LA: 0.95–19.17 mm2); wall area (WA: 2.42–47.02 mm2) and total area (TA: 3.37–66.19 mm2)] covering the range for WA and LA of RB1, in health and in asthma (19) and of airways generations three to 12 (13). Tubes nine to six modelled the RB1 bronchus in health and in asthma across the spectrum of severity, whereas tubes five to one modelled smaller airways down to the 12th generation. The polystyrene had a mean attenuation ±(SEM) of −965 HU (9.3), similar to the density of inflated lung tissue. The range of tube wall attenuation was 154–1029 HU. Gold standard measurements for tube wall and luminal volumes in the phantom airway model were derived using stereomicroscopy with an accuracy of ±1 μm (Olympus SZX12 stereomicroscope; Aquis Pro Software Syncroscopy, Cambridge, UK) (Fig. 1B,C) together with Vernier calipers to measure length and micro-CT. Micro-CT measurements were carried out using a high-resolution CT and digital radiography system (HMXST 225; X-Tek Systems Ltd, Tring, UK) with a tube potential of 50 kV, a copper anode target, a beryllium windowed detector and a 0.5-mm thick aluminium filter to improve image quality by increasing the signal-to-noise ratio. The three-dimensional tomographic volumes were reconstructed using a cone beam extension of the filtered back projection algorithm for fan beams from 370 radiographs acquired using a sample rotation step of 0.5°, with 32 frames averaged for acquisition of each projection (using an exposure time for each frame of 120 ms). Micro-CT measurements served as a second gold standard of tube geometry due to the variability of tube thickness across tube length.
Figure 1. Validation of airway geometry measurements.
(A) A polystyrene phantom with 9 embedded plastic tubes modelling the RB1 and small airways to the 12th generation, (B) a stereomicroscope image of one of the phantom tubes and (C) the corresponding CT image. Correction equations derived from polystyrene phantom for (D) wall and (E) total area derived using the FWHM technique.
A semi-automated program (Emphylyx-J V 1.00.01; British Columbia University, Vancouver, BC, Canada) (20) using the full width half maximum (FWHM) technique was used to determine the accuracy and repeatability of a nonbiased objective measure of edge detection on airway wall cross-sectional geometry. The tube dimensions were measured by three observers. The influence of oblique airway orientation was assessed by reconstructing each phantom tube from 0° (perpendicular to the long axis of the tube) to 60° corresponding to a ratio of the largest to the smallest diameter of 1.0–2.0. Linear correction formulae were derived by comparing the mean geometric measurement of TA and WA for the nine phantom tubes with the gold standard measurements made using stereomicroscopy. The correction equations were used to correct the observed overestimation of WA and under estimation of LA as airway size decreases.
A sheep airway model was used to validate the error equations generated from the phantom model. Samples of lung from a macroscopically healthy sheep lung obtained from a local slaughterhouse were snap frozen at −80°C for 4 h. These samples were imaged using the above CT protocol, and 29 airways TA median (range) [14.0 (4.1–31.9) mm2], LA median (range) [3.5 (0.86–15.5) mm2] and %WA median (range) [69.9 (44.1–89.7) %] were identified and were measured using the FWHM technique and stereomicroscopy.
CT assessment of airway geometry
All airways visible on the CT images from the subjects with asthma, EB and healthy controls were identified and measured using the FWHM technique by two blinded observers. The RB1 bronchus was tracked from its origin to its point of division, and measurements were taken at 0.75-mm intervals (13, 19). The mean of the measurements of WA, LA were corrected for body surface area (BSA) (21). The TA and percentage WA (%WA) were derived from the LA and WA. RB1 length was measured using the mimics© software package (Materialise, Leuven, Belgium). All other visible non-RB1 airways (generations three to six) were measured and corrected for BSA and the error of the analysis algorithm using the linear correction algorithm derived from the phantom airway model.
Assessment of radiation exposure
Dosimetry calculations were performed to quantify the effective dose (mSv) of radiation based upon our limited CT scanning protocol and our Siemens Sensation 16 scanner. Effective dose was calculated using the ImPACT CT dosimetry calculator (Version 0.99x; ImPACT, St. George’s Healthcare NHS Trust, London, UK) with dose distribution data derived using the Monte Carlo approximation and limited scan region estimated based upon the programs mathematical hermaphrodite phantom. We also estimated the effective dose by using a simplified estimate that minimizes method specific difference from reported values, does not require anatomical localization to a phantom and can be derived from the simple equation E = k × DLP (22, 23), where DLP is the dose length product on the scanner console (Gy × cm) and k is a conversion coefficient (mSv × mGy−1 × cm−1) that varies according to body region scanned (k: chest = 0.014 mSv × mGy−1 × cm−1). The estimation of E by this method generally varies by <15% of more complex calculations based upon the Monte Carlo approximation (23).
Statistical analysis
Statistical analysis was performed using prism version 4 (PRISM, San Diego, CA, USA) and regression analysis using spss version 13.0 (SPSS Inc., Chicago, IL, USA). To have an 80% power at the 5% level, to detect a difference between groups in %WA of 7.5% assuming a SD of 6% (13, 19), we estimated that 10 subjects would be required in each group. Normally distributed data were expressed as mean (SD), log normally distributed data was described as geometric mean (95% CI) and non-normally distributed data were described as median (range). One-way anova with Bonferronis correction (normally distributed data) and Dunns intergroup comparison (non-normally distributed data) was used to compare multiple groups. Student’s t-tests and chi-squared tests were used for between group comparisons (two groups) and categorical data respectively. Intra-class correlation (ICC) was used to assess agreement between observers and techniques for airway measurements. A P-value of <0.05 was taken as statistically significant.
Results
Validation of airway geometry measurements
The repeatability between observers for measurements of WA, LA and TA (T1–9) and also between observers and the stereomicroscope measurements was excellent ICC = 1. The LA and WA of the phantom tubes at >50° oblique orientation (equivalent to a maximum/minimum diameter of >1.5) was significantly different between FWHM and stereomicroscope measurements. We therefore excluded airways that had a maximum/minimum diameter of >1.5 from analysis. All airways <6.61 mm2 TA (uncorrected for BSA) were also excluded from airway analysis as this was below the limit of accuracy. There was a close correlation between mean airway geometry using micro-CT and stereomicroscopy of the leading face of phantom tubes one to nine for the wall thickness (r2 = 0.99) and LA (r2 = 0.99). Comparison of the TA and WA obtained by stereomicroscopy for the phantom tubes and the FWHM technique yielded an excellent linear relationship, which allowed us to derive linear correction equations for WA and TA to be applied to the measurement of generation three to six airways (Fig. 1D,E]. In the sheep ex vivo model, a similar linear relationship was found for WA (R2 = 0.93; P < 0.0001) and TA (R2 = 0.96; P < 0.0001). After correction for size-dependant error and exclusion of oblique airways, the total number of non-RB1 airways analysed was 386 across the four groups. The mean (SD) number of non-RB1 airways measured in each patient was 8.5 (3.7).
CT assessment of airway geometry
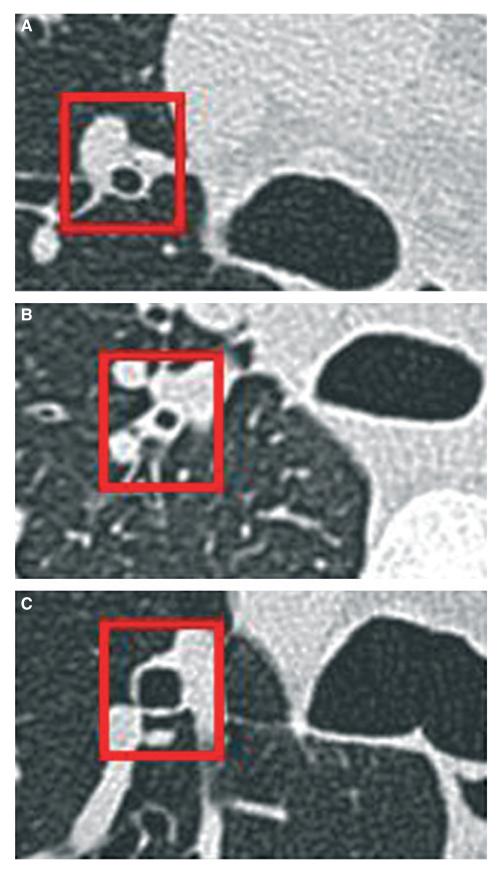
The subjects’ baseline clinical characteristics are as shown (Table 1). Example CT images of the origin of the RB1 in a subject with asthma, EB and a healthy control are as shown (Fig. 2). Measurements of the %WA of the RB1 bronchus were highly repeatable between observers rs = 0.8; P < 0.0001. The airway geometry of RB1 assessed by CT in the subjects with mild-to-moderate asthma or EB was not significantly different before or after treatment with oral corticosteroids. In contrast, sputum and blood eosinophil counts in asthma and sputum eosinophils and eNO in EB were attenuated (Table 2).
Table 1. Baseline demographics.
| Control (n = 11) | Mild-to-moderate asthma (n = 12) | Refractory asthma (n = 14) | Eosinophilic bronchitis (n = 10) | Significance | |
|---|---|---|---|---|---|
| Age (years) | 60.1 (10.4) | 46.3 (18.0) | 51.3 (8.1) | 57.1 (12.3) | ns* |
| Gender M : F | 7 : 4 | 8 : 4 | 9 : 5 | 5 : 5 | ns‡ |
| Atopy (%) | 36 | 58 | 57 | 60 | ns‡ |
| Disease duration (years) | NA | 22.8 (17.0) | 21.7 (16.9) | 10.6 (10.9) | ns* |
| BMI (kg/m2) | 25.4 (3.6) | 27.9 (3.6) | 29.1 (7.4) | 26.5 (6.4) | ns* |
| BDP equivalent (mcg/24 h) | 0 692 (748) | 1829 (760) | 1460 (776) | P = 0.002* | P < 0.01† |
| Oral CS (mg/24 day): n | 0 | 0 | 9.6 (4.7):14/14 | 0 | – |
| Total IgE (KU)§ | 20 [9–44] | 106 [36–314] | 169 [72–395] | 116 [56–242] |
P = 0.004* P < 0.05 vs control |
| Peripheral blood eosinophils§ | 0.14 [0.08–0.24] | 0.16 [0.1–0.25] | 0.27 [0.16–0.44] | 0.29 [0.17–0.47] | ns* |
| FEV1% postbronchodilator | 106.1 (13.8) | 87.4 (21.6) | 79.9 (29) | 108.4 (15.4) |
P = 0.0047* (P < 0.05 EB/control vs refractory asthma) |
| FEV1/FVC | 75.4 (6.2) | 72.4 (10.4) | 65.5 (22.5) | 79.8 (10.3) | ns* |
| %Bronchodilator response FEV1 | 0.44 (6.2) | 9.4 (20.2) | 10.6 (17.4) | 4.4 (5.4) | ns* |
| PC20 (mg/ml)§ | >16 | 0.26 [0.1–0.72] | 2.9 [0.17–48] | >16 | P < 0.0001* |
| Sputum eosinophils %§ | 0.42 [020–0.88] | 1.2 [0.31–4.3] | 5.6 [1.7–18.1] | 4.0 [1.2–13.3] |
P = 0.004 P < 0.05 EB/severe asthma vs control |
| Sputum neutrophils % | 67.8 (26.9) | 49.1 (31.3) | 55.4 (30.5) | 52 (24.0’ | ns |
| eNO (50 mls/s) ppb§ | 16.5 [12.4–21.0] | 20.5 [12.5–33.7] | 31 [15.8–62.0] | 34.5 [22.7–53.6] | ns* P = 0.07 |
Data expressed as mean (SD). Intergroup comparisons: t-test and one-way ANOVA with Bonferroni correction for multiple comparisons.
BMI, body mass index; BDP, beclamethasone diproprionante equivalents; fluticasone 2 : 1, budesonide 1.25 : 1, mometasone 1 : 1; CS, corticosteroid; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; eNO, exhaled nitric oxide.
ANOVA.
Mild asthma vs severe asthma.
Chi-squared test.
Geometric mean [95% CI].
Figure 2. Example CT images of the RB1.
Postprednisolone images illustrating (A) a healthy control with an absence of airway wall thickening and preserved luminal calibre, (B) a refractory asthmatic with airway wall thickening and narrowing of the airway lumen and (C) a subject with EB with maintained patency of the airway lumen without evidence of wall thickening (×5 magnification).
Table 2. The effect of 2 weeks of prednisolone on measures of inflammation and airway geometry in asthma and EB.
| Mild-to-moderate asthma (n = 11) |
EB (n = 10) |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | P-value | Pre | Post | P-value | |
| Inflammation | ||||||
| Sputum eosinophils %* | 1.15 [0.3–4.3] | 0.66 [0.2–1.8] | 0.49 | 4 [1.2–13.3] | 1.2 [0.5–2.8] | 0.01 |
| Blood eosinophils* | 0.17 [0.1–0.27] | 0.06 [0.03–0.13] | 0.03 | 0.29 [0.18–0.47] | 0.18 [0.1–0.33] | 0.22 |
| eNO 50 mls/s (ppb)* | 20.4 [11.8–35.3] | 18 [12.1–26.8] | 0.38 | 34.9 [22.7–53.6] | 22.6 [15.6–32.7] | 0.03 |
| RB1 geometry | ||||||
| WA/BSA mm2/m2 | 12.1 (3.0) | 13.16 (2.7) | ns | 16.14 (6.3) | 17.08 (4.6) | ns |
| LA/BSA mm2/m2 | 6.6 (3.4) | 6.56 (2.8) | ns | 10.99 (5.1) | 11.05 (4.6) | ns |
| TA/BSA mm2/m2 | 18.67 (6.2) | 19.73 (5.0) | ns | 27.12 (10.3) | 28.12 (8.6) | ns |
| %WA | 66.4 (7.5) | 67.7 (7.3) | ns | 60.0 (7.8) | 61.4 (7.8) | ns |
Mean (SD); paired t-tests for pre- and postprednisolone comparisons for RB1.
eNO, exhaled nitric oxide; RB1, right upper lobe apical segmental bronchus; WA, wall area; BSA, body surface area; LA, luminal area; TA, total area. Geometric mean [95% CI].
Geometric mean [95% CI].
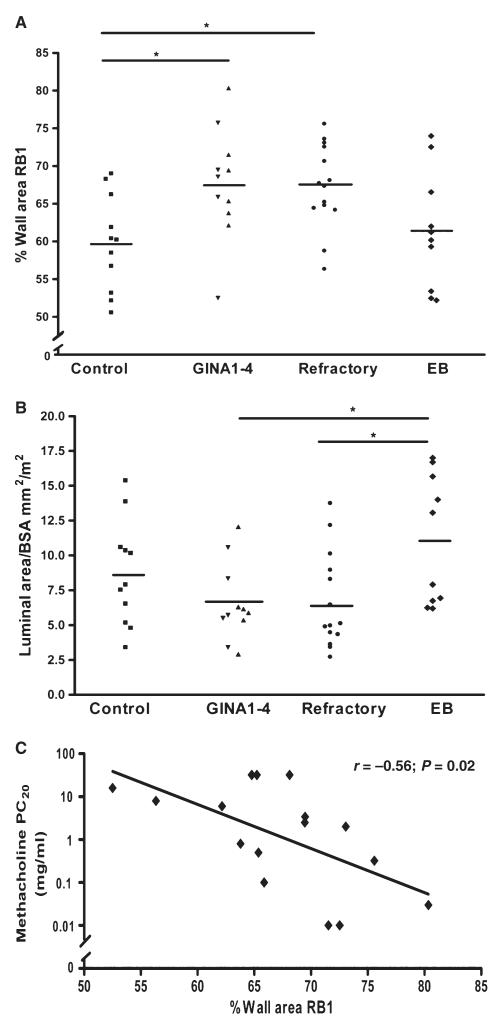
The mean (SD)% WA of RB1 postprednisolone was significantly greater in mild-to-moderate persistent asthma [67.7 (7.3)%] and refractory asthma [67.3 (5.6)%] compared to the control group [59.7 (6.3)%], but not to the EB cohort [61.4 (7.8)%]; P = 0.01 (Fig. 3A). The mean (SD) LA (mm2/m2) of the RB1 bronchus postprednisolone was significantly greater in EB 11.1 (4.6) compared to subjects with mild-to-moderate asthma 6.6 (2.8) and refractory asthma 6.7 (3.4), but not to the control group 8.7 (3.8); P = 0.02 (Fig. 3B).
Figure 3. Airway wall thickening in asthma.
(A) %WA and (B) luminal area/BSA of RB1 postprednisolone in subjects with asthma, EB and healthy controls and (C) the correlation of %WA of RB1 and methacholine PC20 in the pooled refractory and mild-to-moderate asthma cohort. *P < 0.05.
The measurement of airway length of RB1 was highly repeatable between observers (rs = 0.86; P < 0.0001) and within observer (rs = 0.8; P < 0.0001). The mean (SD) length of the RB1 bronchus was similar in all groups; 8.2 (2.9) mm controls, 8.2 (2.1) mild-to-moderate persistent asthma, 7.8 (2.8) mm refractory asthma and 6.9 (2.1) mm in EB; P = 0.63.
The %WA of RB1 demonstrated a significant inverse correlation with the log methacholine PC20 in the pooled asthma cohort postprednisolone (r = −0.56; P = 0.02) (Fig. 3C), but not the bronchodilator response %FEV1 (−0.16; P = 0.44), postbronchodilator FEV1% (r = −0.16; P = 0.44) or log sputum eosinophils (r = −0.09; P = 0.69).
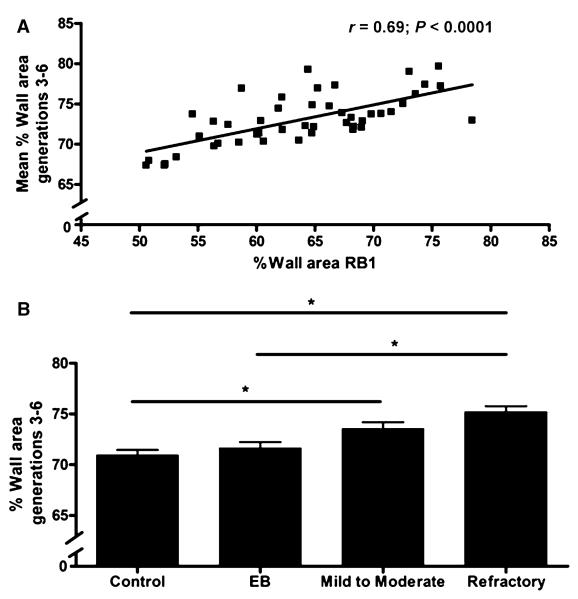
There was a significant correlation between the %WA of RB1 and the mean %WA of non-RB1 airways generations three to six in the pooled asthma cohort (r = 0.47; P = 0.02), EB (r = 0.78; P = 0.008) and healthy subjects (r = 0.81; P = 0.0025) (Fig. 4A). In the more distal airways (generations three to six), the mean (SEM) %WA was significantly increased in the subjects with refractory asthma [75.2 (5.2)%], and mild-to-moderate persistent asthma [73.6 (5.5)%] compared with the control group [70.9 (5.2)%] and between refractory asthma and the EB cohort [71.7 (6.2)%] (P < 0.0001) (Fig. 4B).
Figure 4. Airway wall thickening in non-RB1 airways generations 3–6 in asthma.
(A) Correlation of RB1 %WA and non-RB1 airways generations 3–6 in whole cohort, (B) corrected %WA [mean (SD)] of non-RB1 airways (generations 3–6) in subjects with asthma, EB and healthy controls. *P < 0.05.
Limited CT of the apical bronchus is a low radiation procedure
Using the Monte Carlo approximation, the effective radiation dose from a single limited CT was 0.42 mSv. The effective dose, based upon the approximation from the scanner console derived DLP, was (32 × 0.014) = 0.45 mSv.
Discussion
We have shown for the first time that the subjects with EB have maintained patency of the right upper lobe apical bronchus (RB1), a third generation proximal airway and other conducting airways generations three to six without evidence of airway wall thickening. In contrast, subjects with corticosteroid treated asthma had evidence of airway wall thickening in RB1 and smaller airways, and reduced luminal patency compared to EB. There was a strong correlation between proximal airway wall thickening and AHR in asthma. Therefore, in contrast to EB there is CT evidence of airway remodelling in asthma, which is related to AHR.
We chose to use CT as a measure of airway remodelling as it provides a global measure of wall geometry that cannot be derived from endobronchial biopsies. Indeed CT has emerged as a useful tool to study airway wall remodelling in asthma and chronic obstructive pulmonary disease (COPD) (19, 20). Indeed, a recent study has suggested that airway wall geometry of RB1 correlated well with non-RB1 proximal airways, which averaged over 19 bronchial segments in severe asthma (27). Using a phantom and ex vivo sheep model and applying stereomicroscopy and micro-CT as ‘Gold’ standard measurements, we have demonstrated that airway geometry can be measured accurately. We found that the geometric measurements were highly repeatable between observers and that correction equations can be derived from phantom studies to adjust for size-dependant bias in airway geometry due to partial volume averaging, as airways become smaller.
Using CT we have demonstrated that AHR is related to the thickening of the proximal airway wall in asthma. This view is strengthened by the absence of airway wall thickening in EB and is in keeping with a recent report in severe asthma (27). Our findings are supported by mathematical models that suggest thickening of the airway wall is fundamental in the development of AHR in asthma (28) and underpins the view that AHR is a consequence of remodelling (29). In contrast, Niimi et al. (19) have demonstrated that airway reactivity is inversely proportional to the thickness of the RB1. One possible explanation of this apparent anomaly is that airway reactivity and AHR may be linked to different mechanical properties of the airway wall. The LA was not different between subjects with asthma and healthy controls as described previously (13, 24–30), but was reduced compared to subjects with EB. This maintained airway patency of the proximal airway lumen in EB may contribute to the absence of AHR in this disease control model. Interestingly, in bronchiectasis proximal dilatation is protective against airflow limitation (31). Our findings extend the earlier observations by Park et al. (11) who described the absence of proximal airway wall thickening in EB together with evidence of distal disease as evidenced by centrilobular opacification within secondary pulmonary lobules and air trapping in expiratory CT scans. The presence of distal airway disease may account for why some patients with EB develop fixed airflow obstruction (32). However, we chose EB subjects with normal lung function with repeated measures over several years and therefore are likely to have excluded the important subgroup of EB subjects that develop airflow obstruction.
In comparative studies of EB and asthma, we have reported that increased vascularity, reticular basement membrane thickening and increased airway smooth muscle mass are features of both diseases (2, 9, 10). These earlier findings suggest that remodelling and AHR are dissociated. However, our current study provides evidence that AHR and altered airway geometry are correlated. One possible explanation for this apparent paradox is that remodelling occurs in both diseases, but in asthma the remodelling process leads to airway wall thickening and relative luminal narrowing compared to EB, whereas in EB the remodelling occurs away from the lumen leading to relative airway dilatation and an increase in the TA of the airway compared with the asthmatic subjects. An alternative or perhaps synergistic mechanism to explain the difference in airway function between asthma and EB is the difference in localization of mast cells within the airway wall. We and others have demonstrated that the mast cells are microlocalized within the airway smooth muscle bundle in asthma and that this is associated with AHR [reviewed in (33)].
We were unable to demonstrate any change in static geometry in mild-to-moderate asthma or EB after a 2-week trial of oral prednisolone despite improvement in markers of airway inflammation. In contrast, Matsumoto et al. (34) have shown that treatment with inhaled budesonide led to a significant reduction in airway wall thickening of RB1 after 12 weeks in asthma without any reduction in wall calibre after a median follow-up of 4.2 years. This would suggest that the anti-remodelling effects of corticosteroids may have a latency of between 2 and 12 weeks. Long-term interventional studies of novel therapies with CT imaging as an outcome measure are required.
We performed dosimetry calculations to estimate the radiation effective dose from our limited CT protocol. We found that a single limited CT was equivalent to one-fifth of the typical dose accrued from a standard high resolution computed tomography scan performed at a UK centre (35) and about one-fifth of the annual natural background radiation in the UK. Similarly, the radiation dose of a limited scan was substantially less than that reported in the severe asthma research program, which was up to 7.6 mSv/scan (36). We are therefore confident that our limited CT protocol is a low-dose protocol and could therefore potentially be used serially in the assessment of airway wall thickening in adults.
One limitation of our study is its cross-sectional design. We therefore do not have longitudinal data to support the notion that the increased luminal and total airway area in those subjects with EB compared to asthma truly represents remodelling rather than an innate geometric airway advantage. However, from longitudinal case series we recognize that some subjects with EB can develop fixed airflow obstruction supporting the view that remodelling does occur in EB (32). Another potential shortcoming is that we did not use respiratory gating to gate CT scans to full inspiration. However, animal studies have shown that there are negligible effects of lung volume on static airway calibre at transpulmonary pressures >10 cm H2O (37), suggesting that spirometric gating is unlikely to be necessary if a good respiratory effort is made. Mathematical models have also demonstrated that smooth muscle tone affects airway narrowing (38, 39). However, we are confident that this did not influence the differences we observed between asthma and controls as all of the moderate-severe asthma subjects were treated with a long-acting bronchodilator 2 h prior to their CT scan.
In summary, we have shown that airway wall geometry is differentially altered in asthma and EB. Maintained proximal airway patency in EB compared to the subjects with asthma may protect against the development of AHR, whereas airway wall thickening may promote AHR in asthma.
Acknowledgments
We thank Mrs S. McKenna for assistance in clinical characterization, Mr W. Monteiro for technical support in sputum processing, Dean Mawby for coordinating research CT scans and Materialize for access to their mimics© software.
Funding: Asthma UK, DoH Clinician Scientist award (CB) and Wellcome Senior Clinical Fellowship (CB).
References
- 1.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui S, Sutcliffe A, Shikotra A, Woodman L, Doe C, McKenna S, et al. Vascular remodelling is a feature of asthma and non-asthmatic eosinophilic bronchitis. J Allergy Clin Immunol. 2007;120:813–819. doi: 10.1016/j.jaci.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 4.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172:837–841. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 5.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 6.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–1109. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 8.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl.):116S–121S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- 9.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui S, Mistry V, Doe C, Roach K, Morgan A, Wardlaw A, et al. Airway hyper responsiveness is dissociated from airway wall structural remodelling. J Allergy Clin Immunol. 2008;122:335–341. doi: 10.1016/j.jaci.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SW, Park JS, Lee YM, Lee JH, Jang AS, Kim DJ, et al. Differences in radiological/HRCT findings in eosinophilic bronchitis compared to asthma: implication for bronchial responsiveness. Thorax. 2005;61:41–47. doi: 10.1136/thx.2005.044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui S, Brightling CE. Differences in airway wall remodelling in asthma and EB. Thorax. 2006;61:547. [PMC free article] [PubMed] [Google Scholar]
- 13.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography – relation to clinical indices. Am J Respir Crit Care Med. 2000;162:1518–1523. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma [last accessed 12 December 2006];Global initiative for asthma guidelines 11/06. 2007 http://www.ginasthma.com
- 15.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 16.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Cockcroft DW, Hargreave FE. Histamine and methacholine inhalation tests: a laboratory tidal breathing protocol. 2nd edn Astra Draco AB; Lund: 1994. [Google Scholar]
- 18.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003;168:983–988. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 20.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 21.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 22.Mayo JR, Aldrich J, Muller NL. Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology. 2003;228:15–21. doi: 10.1148/radiol.2281020874. [DOI] [PubMed] [Google Scholar]
- 23.Gerber TC, Kuzo RS, Morin RL. Techniques and parameters for estimating radiation exposure and dose in cardiac computed tomography. Int J Cardiovasc Imaging. 2005;21:165–176. doi: 10.1007/s10554-004-5338-6. [DOI] [PubMed] [Google Scholar]
- 24.Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J. 2003;22:965–971. doi: 10.1183/09031936.03.00085302. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 26.Nakano Y, Muller NL, King GG, Niimi A, Kalloger SE, Mishima M, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122:271S–275S. [PubMed] [Google Scholar]
- 27.Aysola RS, Hoffman EA, Gierada D, wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector computed tomography is increased in severe asthma and correlates with pathology. Chest. 2008;134:1183–1191. doi: 10.1378/chest.07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 29.Pare PD, Bai TR, Roberts CR. The structural and functional consequences of chronic allergic inflammation of the airways. Ciba Found Symp. 1997;206:71–86. doi: 10.1002/9780470515334.ch5. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto H, Niimi A, Tabuena RP, Takemura M, Ueda T, Yamaguchi M, et al. Airway wall thickening in patients with cough variant asthma and nonasthmatic chronic cough. Chest. 2007;131:1042–1049. doi: 10.1378/chest.06-1025. [DOI] [PubMed] [Google Scholar]
- 31.Roberts HR, Wells AU, Milne DG, Rubens MB, Kolbe J, Cole PJ, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198–204. doi: 10.1136/thorax.55.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry MA, Hargadon B, McKenna S, Shaw D, Green RH, Brightling CE, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598–601. doi: 10.1111/j.1365-2222.2005.02222.x. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui S, Hollins F, Saha S, Brightling CE. Inflammatory cell microlocalisation and airway dysfunction: cause and effect? Eur Respir J. 2007;30:1043–1056. doi: 10.1183/09031936.00162506. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto H, Niimi A, Ueda T, Takemura M, Yamaguchi M, Matsuoka M, et al. Long-term changes of airway wall thickness as assessed by computed tomography (CT) in asthmatic patients taking inhaled corticosteroids (ICS) Am J Resp Crit Care Med. 2007 A332 (Abstract) [Google Scholar]
- 35.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. Doses from computed tomography (CT) examinations in the UK – 2003 review. National Radiation Protection Board (NRPB)-W67; Chilton: 2005. [Google Scholar]
- 36.Busacker A, Newell JD, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RH, Mitzner W. Effect of lung inflation and airway muscle tone on airway diameter in vivo. J Appl Physiol. 1996;80:1581–1588. doi: 10.1152/jappl.1996.80.5.1581. [DOI] [PubMed] [Google Scholar]
- 38.Wiggs BR, Bosken C, Pare PD, James A, Hogg JC. A model of airway narrowing in asthma and in chronic obstructive pulmonary-disease. Am Rev Respir Dis. 1992;145:1251–1258. doi: 10.1164/ajrccm/145.6.1251. [DOI] [PubMed] [Google Scholar]
- 39.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis. 1986;133:1171–1180. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]