Abstract
Minocycline (MCN), a semi-synthetic tetracycline derivative possesses pleiotropic effects and provides protection against a number of disease models. However its effect on gastric ulcers has not been studied. The present investigation was undertaken, to study the gastro-protective potential of MCN in experimentally induced gastric ulcers in rats. MCN (10, 30, 100 mg/Kg) was tested for gastric secretion and antiulcer activity in different groups of Wistar rats. Gastric secretion and acidity studies were performed in pylorus ligated rats while indices of gastric ulcers were measured in ethanol (1 ml-100%) and indomethacin (30 mg/kg), induced gastric ulcers. Histological changes and the levels of gastric wall mucus, malondialdehyde (MDA), non-protein sulfhydryl (NP-SH), and myeloperoxidase (MPO), were used to assess ethanol induced gastric mucosal injuries. Exposure of rats to ulcerogens resulted in gastric mucosal injury and a significant increase in the indices of ulcer. MCN conferred a protective effect against ethanol, and indomethacin induced gastric mucosal injuries. Treatment with MCN, resulted in a significant decrease in the amount of gastric secretion, and total acidity and significantly (P<0.001), reduced the gastric lesions induced by ethanol and indomethacin. MCN also significantly attenuated the ethanol induced reduction in the levels of gastric wall mucus, and NP-SH (P<0.001). The histological changes and the increased MDA and MPO activity were also significantly (P<0.001) inhibited by MCN. Minocycline showed significant antiulcer and gastroprotective activity against experimentally induced gastric ulcers. The gastroprotective effects of minocycline may be due to its anti-secretory, antioxidant and anti inflammatory action.
Keywords: Gastric ulcer, ethanol, indomethacin, minocycline, oxidative stress, myeloperoxidase
Introduction
Gastric ulcers predominantly characterized by the damage to the gastric mucosa, is a disease with multifactorial etiology. The predisposing factors identified for gastric ulcerogenesis include bacterial infection, excess intake of alcohol, stress, use of steroidal and non-steroidal anti-inflammatory medication, nutritional deficiencies and trauma [1,2]. Gastric ulcers have also been suggested to occur due to an imbalance between the levels of defensive factors and destructive injurious by products in the gastric mucosa [3,4]. Oxidative stress, depletion of antioxidants, neutrophil accumulation, increase in inflammatory cytokines, and matrix metalloproteinase activity, and reduced blood supply to the gastric mucosa have all been implicated in the pathophysiology of gastric ulcers [5-9]. Exposure to ulcerogens results in excessive production of reactive oxygen species (ROS) which are harmful for the gastric mucosa [10], whereas the mucus layer and endogenous antioxidants which are part of the gastrointestinal defense help in the protection against ROS induced cytotoxicity [7,11]. The infiltration of activated neutrophils also results in the excessive generation of ROS which leads to disturbances in the gastric mucosal microcirculation [12]. Neutrophil infiltration has also been suggested to be a critical component in the development of gastric ulcers. The enzyme myeloperoxidase is used as an indicator of neutrophil infiltration in gastric ulcer pathogenesis [7,9,13-15].
In spite of the large number of drugs available for the treatment of gastric ulcers, adverse effects of these drugs necessitate a constant search for new antiulcer therapies. Moreover, gastric ulcer being a multi etiological disease, drug/s with multiple modes of actions may be helpful in attenuation of gastric ulcers. Minocycline a semi-synthetic tetracycline derivative possesses biological actions which are significantly different from its antimicrobial effects. The pleiotropic effects of MCN include, free radical scavenging activity [16,17], anti-inflammatory action, and inhibition of matrix metalloproteinases [18-20]. MCN through a combination of its antioxidant, anti-apoptotic, anti-inflammatory and immunomodulatory effects was recently observed to provide protection in animal models of intestinal disorders [21-24]. Concurrently, doxycycline another tetracycline derivative was observed to arrest chemically induced gastric damage in rats through its antioxidant properties, and regulation of matrix metalloproteinases [20]. Though minocycline and doxycycline are both tetracyclines, the differences in the pharmacokinetics and adverse effects between them are likely to have a variation in their comparative efficacy [25]. Furthermore, MCN has been reported to be clinically useful in treating peptic disease caused by Helicobacter pylori [26,27]; however, it has not been studied in gastric ulcers. Therefore, this study was undertaken to investigate the gastro protective and antiulcer potential of MCN against chemically induced gastric ulcers in rats.
Materials and methods
Approval for the study was obtained from the institutional research and ethics committee. The institutional guidelines for animal care were strictly observed for animal maintenance and during experimentation.
Wistar strain rats of either sex were used in the study. Rats weighing between 150-200 g were randomly distributed into different groups of six animals each. Minocycline in doses of 10, 30 and 100 mg/kg body weight was given intraperitoneally (i.p.) for gastric secretion studies and orally by intubation needle for antiulcer activity. Each rat was administered the desired concentration of the drug in a volume of 0.5 ml/100 g body weight.
Gastric secretion studies
Gastric secretion studies were performed in different groups of pylorus ligated rats as described by Shay et al [28]. Pylorus ligation was performed under anesthesia. Before ligation, the animals were deprived of food for 36 hrs with unlimited access to water. Necessary precautions were taken, to prevent bleeding or the occlusion of blood vessels. Minocycline in doses of 10 mg, 30 mg, and 100 mg/kg was injected intraperitoneally to the different groups of rats immediately after the ligation of the pylorus. The animals were killed at 6 h following pylorus ligation. The contents of the stomachs were emptied, and the collected volume was measured, centrifuged and analyzed for acidity using 0.01N NaOH.
Indomethacin induced gastric lesions
Indomethacin (30 mg/Kg body weight) was given orally (i.g.) for inducing gastric lesions in rats [29]. Minocycline in doses of 10 mg, 30 mg, and 100 mg/kg was administered orally 1 h before giving indomethacin. The animals were killed under deep anesthesia 6 h following the administration of indomethacin.
The stomachs were collected, opened, and washed with saline and the gastric lesions were measured blindly to determine the ulcer index. The scoring of ulcers was based on the size and was performed as described by Valcavi et al. [30].
Ethanol induced gastric lesions (cytoprotection studies)
The gastroprotective potential of minocycline was assessed against ethanol (1 ml of 100% ethanol) induced gastric mucosal damage by following the method of Natale et al [31]. Minocycline was given orally (i.g.) in doses of 10 mg. 30 mg and 100 mg/kg 30 minutes before the ethanol was administered. The animals were killed 1 h after giving ethanol and the gastric lesions were scored by the method of Schiantarelli et al [32].
Separate batches of ethanol treated rats were used for biochemical and histological studies. Minocycline (10 mg, 30 mg and 100 mg/Kg), was administered 30 minutes before ethanol administration. The animals were killed 1 h after administering ethanol. The stomachs were collected and the levels of gastric wall mucus, nonprotein sulfhydryl group (NP-SH), malondialdehyde, and myeloperoxidase (MPO) were determined.
Determination of gastric wall mucus
A modified procedure of Corne et al [33] that employs the Alcian blue binding capacity of the gastric wall was used for the determination of gastric wall mucus. The glandular part of the stomach was isolated from the inner wall of the stomach, weighed and stained for two hours in a 10 ml solution of 0.1% Alcian blue prepared in buffered (pH 5) solution of sucrose. At the end of two hours, the excess dye was removed by rinsing twice in 0.25 M sucrose solution. The dye bound to the gastric wall mucus, was then obtained by extraction with 10 ml solution of 0.5 M magnesium chloride by intermittent shaking for 2 hrs. 4 ml of the extract were thoroughly mixed with 4 ml of diethyl ether and centrifuged for 10 minutes (3600 rpm). The aqueous layer was separated and the absorbance was recorded at 580 nm on a UV spectrophotometer. The amount of Alcian blue extracted was then calculated.
Determination of nonprotein sulfhydryl groups (np-sh) of the gastric mucosa
NP-SH levels in the gastric mucosa were determined by following the method described by Sedlak and Lindsay [34]. Homogenates of the isolated glandular part of the stomach were prepared in ice-cold 0.02 M mmol/L EDTA. Aliquots of the of the homogenates (5 ml) were mixed with distilled water (4 ml) and 50% trichloroacetic acid (1 ml) in a test tube and the mixture was centrifuged at 3000 g after shaking intermittently for 15 minutes. The supernatant was collected and 2 ml of the supernatant were added to 4 ml of 0.4 M Tris buffer (pH 8.9) and 0.1 ml of DTNB [5, 5’-dithio-bis-(2-nitrobenzoic acid)]. The mixture was shaken, and the absorbance was read at 412 nm against a reagent blank with no homogenate. Care was taken to record the absorbance in less than 5 minutes after adding DTNB.
Determination of malondialdehyde (MDA)
Gastric mucosal malondialdehyde an indicator of lipid peroxidation was determined by the method of Ohkawa et al [35]. Pre weighed gastric mucosa was homogenized in 1.15% KCL. For the assay of MDA 100 μl of homogenates was added to 200 μl of 8.1% sodium dodecyl sulfate, and 1500 μl of 0.8% aqueous solution of thiobarbituric acid. The test tubes were placed in a water bath at 95°C for 1 h and cooled at room temperature for 15 minutes. After cooling, the contents of the test tube were mixed with 1.0 ml of sterile distill water and 5 ml of a mixture of n-butane and pyridine (in a 15:1 ratio v/v) and centrifuged at 4000 rpm for ten minutes. The upper layer was separated and the absorbance was measured at 532 nm against TBA as a blank on a UV visible Spectrophotometer (UV-160A, Shimadzu, Japan). Results were calculated as nmol TBARS formed/h/gm tissue using an extinction coefficient of 1.56×106.
Determination of myeloperoxidase (MPO)
The method as described by Bradley et al [36] was used to determine the gastric mucosal MPO activity. The homogenates were subjected to three cycles of freezing and thawing and sonicated in an ice bath for 20 seconds. The supernatant collected after centrifugation of samples was assayed for MPO activity.
Histological analysis of ethanol-induced gastric lesions
The stomachs were cut and washed with saline and then fixed in 10% neutral buffered formalin for 24 h. The tissues were processed and stained with hematoxylin-eosin. The slides were examined for the histological changes under a light microscope.
Statistical analysis
The values are presented as ± SEM. Analysis of variance and Dunnett’s multiple comparison tests were used to analyze the difference between the groups. A P value of <0.05 was considered as significant. The statistical program SPSS (Version 15) was used to analyze the data.
Results
Effect of MCN on gastric secretion and acidity in 6 h pylorus-ligated shay rats
The results of this study show for the first time, that minocycline has significant anti-gastric acid secretory activity (Table 1). Pylorus ligation for 6 h resulted in the accumulation of 9.0±0.7 ml of gastric secretion in control rats. Treatment of rats with 10, 30 and 100 mg/kg of minocycline significantly reduced the volume of gastric secretion to 5.2±0.37 ml, 5.8±0.37 ml and 5.8±1.15 ml respectively as compared to the control rats (ANOVA, F=5.60 P<0.008). The total acid output in control rats was 614±68.8 mEq. Total acid output was significantly reduced in rats treated with minocycline at 10 mg/kg (238±18.5 mEq), 30 mg/kg (306±25.2 mEq) and 100 mg/kg (290±73.1 mEq) (ANOVA, F=10.516 P<0.001).
Table 1.
Effect of Minocycline (MCN) on gastric secretions and acidity in 6 h pylorus ligated (shay) rats
| Treatment | Volume of gastric secretion (ml) | Total acid output (mEq) |
|---|---|---|
| Control (Pylorus ligation only) | 9.0±0.7 | 614±68.8 |
| Ligation + MCN 10 mg/kg | 5.2±0.37** | 238±18.5## |
| Ligation + MCN 30 mg/kg | 5.8±0.37* | 306±25.2# |
| Ligation + MCN 100 mg/kg | 5.8±1.15* | 290±73.1# |
Values are mean ± standard error of mean.
P<0.05;
P<0.005.
P<0.005;
P<0.001 as compared with control group using Dunnett’s multiple comparison test.
Effect of MCN on mucosal lesions in the stomach of ethanol treated rats
Treatment of rats with ethanol produced widespread lesions in the gastric mucosa that were significantly attenuated upon pretreatment with minocycline (Figure 1).
Figure 1.
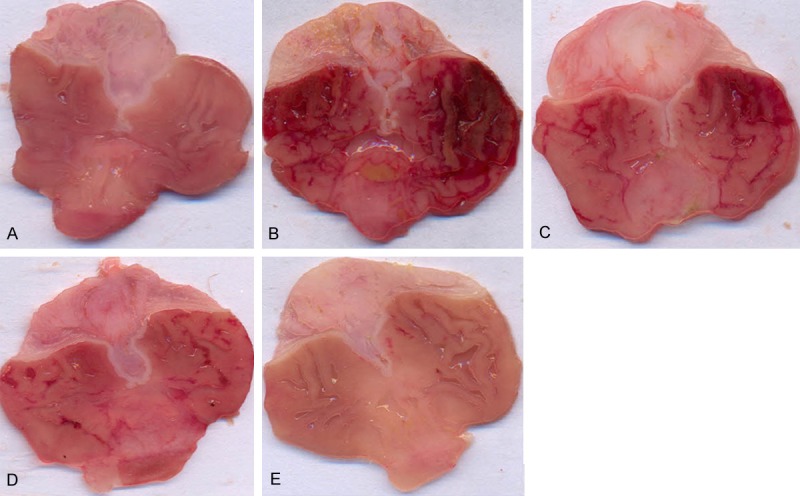
Morphological appearance of hemorrhagic lesions in the stomach of rats treated with (A) Saline only (B) Ethanol only (C) Minocycline 10 mg/kg + ethanol (D) Minocycline 30 mg/kg + ethanol (E) Minocycline 100 mg/kg + ethanol.
The ulcer index in control rats 1 h after ethanol administration was 7.33±0.33. Minocycline at the doses of 10 mg/kg, (ulcer index=5.83±0.30), 30 mg/kg, (2.33±0.21), and 100 mg/kg (0.33±0.21), resulted in a significant inhibition of gastric lesions (ANOVA, F=139 P<0.001, Figure 2).
Figure 2.
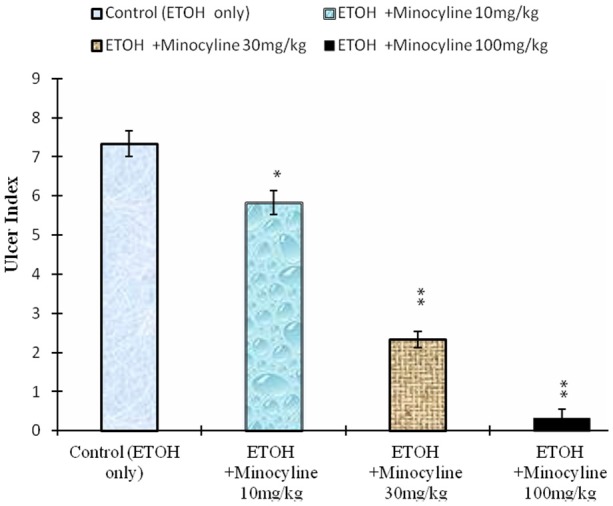
Effect of minocycline on ethanol induced gastric mucosal damage in rats. Values are mean ± SEM. *P<0.005 and **P<0.001 as compared with control using Dunnett’s multiple comparison test. Animals in control group were killed 1 h after the oral administration of ethanol. In the test group minocycline was given by gavage 30 min before the administration of ethanol.
Histological changes
Administration of ethanol to rats resulted in a severe injury to gastric mucosa. The gastric mucosal injury was characterized by numerous hemorrhagic red bands (patches) of various sizes distributed in the glandular stomach (Figure 1). Assessment of the histology of the gastric mucosa showed the presence of hemorrhage, inflammation, gastric lacerations, and infiltration of leucocytes in the sub mucosal layer and the epithelial surface of the mucosa appeared detached with elongation of the microvessels (Figure 3B). Pretreatment with minocycline dose-dependently reduced the size and severity of ethanol induced damage to the gastric mucosa and restored the mucosal architecture (Figures 1 & 3C-E).
Figure 3.
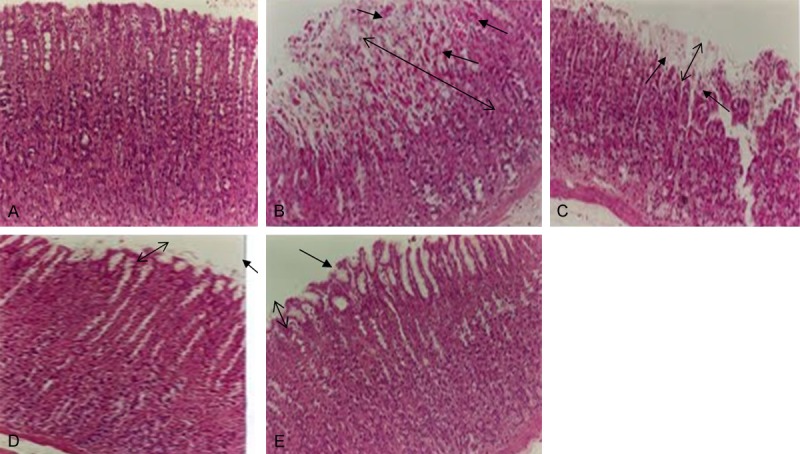
Light micrographs illustrating the effect of pretreatment with minocycline on ethanol induced gastric lesions.A: Stomach from normal rats (saline). B: Stomach from rats administered with ethanol showing breaches in the gastric mucosa with loss of epithelial cells, edema and hemorrhage. C: Stomach of rats pretreated with minocycline 10 mg/kg + ethanol showing less damage to gastric mucosa with slight hemorrhage and edema. D & E: Stomach of rats pretreated with 30 and 100 mg/kg minocycline showing preservation of gastric mucosa and an outline similar to that of normal rats. Arrows indicate the damage and recovery of the gastric mucosa in the ulcer and the treated groups. ↕The double arrow line indicates the reference for mucosal thickness.
Effect of MCN on indomethacin-induced gastric mucosal damage
The effect of treatment with minocycline on indomethacin induced gastric mucosal damage is shown in Figure 4. Indomethacin treatment resulted in production of gastric lesions mainly in the glandular stomach in all the animals. In the control group the size of the lesion was 30±1.75 mm2. Pretreatment of rats with minocycline at doses of 10 mg/kg (10±1.8 mm2), 30 mg/kg (5.6±1.3 mm2), and 100 mg/kg (1.8±.6 mm2), significantly decreased the lesion area (ANOVA F=73.04 P<0.001) (Figure 4).
Figure 4.
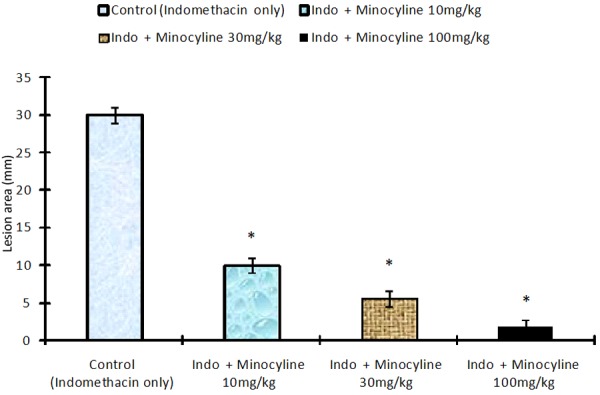
Effect of Minocycline on indomethacin induced gastric mucosal damagein rats. Values are mean ± S.EM. *P<0.001 as compared with control using Dunnett’s multiple comparison test. Animals in control group were killed 6 h after the oral administration 6 h after the oral administration of indomethacin. In the test group minocycline was given by gavage 30 min before the administration of indomethacin.
Effect of MCN on ethanol-induced changes in gastric wall mucus
The results of the ethanol induced changes in the gastric wall mucus of control and minocycline treated rats is shown in Table 2. Alcian blue binding capacity of gastric wall mucus was significantly reduced in the rats exposed to ethanol treatment (2397.2±58.1 μg Alcian blue/g of tissue) as compared to control rats (6678±21 μg/g tissue). Pretreatment of rats with minocycline at the doses of 10 mg/kg (2415.8±44.7 μg/g tissue), 30 mg/kg (2724.8±56.5 μg/g tissue) and 100 mg/kg (3660.5±23.5 μg/g tissue) significantly improved Alcian blue binding capacity of gastric mucosa (ANOVA F=514 P<0.001) (Table 2).
Table 2.
Effect of Minocycline (MCN) on ethanol induced changes in Alcian blue binding capacity (gastric wall mucus) in gastric mucosa of rats
| Treatment | Alcian blue binding (μg/g tissue) |
|---|---|
| Normal | 6678±21 |
| Ethanol alone (EtOH) | 2397.2±58.1* |
| EtOH + MCN 10 mg | 2415.8±44.7 |
| EtOH + MCN 30 mg | 2724.8±56.5# |
| EtOH + MCN 100 mg | 3660.5±23.5## |
Values are mean ± SEM.
P<0.001 as compared with control group.
P<0.05;
P<0.001 as compared with Ethanol (ulcer only) group using Dunnett’s multiple comparison test.
Effect of MCN on ethanol-induced changes in gastric mucosa levels of nonprotein sulfhydryls (NP-SH)
The results of the effect of minocycline on ethanol induced changes in the level of nonprotein sulfhydryls of the gastric mucosa are presented in Table 3. The levels of NP-SH in the gastric mucosa of normal animals were 2.87±0.054 μmol/g tissue and were significantly decreased to 1.73±0.029 μmol/g tissue in ethanol treated rats. Pretreatment of rats with minocycline at 30 mg/kg (2.12±0.084 μmol/g tissue) and 100 mg/kg (2.59±0.024 μmol/g tissue) significantly increased the gastric mucosal NP-SH levels (ANOVA F=9.94 P<0.001).
Table 3.
Effect of Minocycline (MCN) on ethanol induced changes in malondialdehyde (MDA), myeloperoxidase (MPO) and non protein sulfhydryl (NPSH) levels in gastric mucosa of rats
| MDA (nmol TBARS/h/gm tissue) | MPO A/g tissue Δ | NP-SH μmol/g tissue | |
|---|---|---|---|
| Control | 34.58±1.32 | 2.76±0.23 | 2.87±0.054 |
| Ethanol alone (EtOH) | 56.81±1.77* | 4.85±0.31* | 1.73±0.029* |
| EtOH + MCN 10 mg | 52.12±0.97# | 3.82±0.0.41 | 1.75±0.021 |
| EtOH + MCN 30 mg | 44.15±1.32## | 2.37±0.0.28## | 2.12±0.084## |
| EtOH + MCN 100 mg | 35.62±0.70## | 2.76±0.0.23## | 2.59±0.024## |
Values are means ± standard error of mean.
P<0.001 as compared with control group.
P<0.05;
P<0.001 as compared with Ethanol (ulcer only) group using Dunnett’s multiple comparison test.
Effect of MCN on ethanol-induced changes in gastric mucosa levels of malondialdehyde (MDA)
The effect of minocycline on ethanol induced changes in the gastric mucosa MDA levels is given in Table 3. Administration of ethanol resulted in a significant increase in the levels of MDA (56.81±1.77 nmol TBA/g tissue) as compared to the levels in control animals (34.58± 1.32 nmol/g). Pretreatment with minocycline at 10 mg/kg (52.12±0.97 nmol/ g), 30 mg/kg (44.15±1.32 nmol/g) and 100 mg/kg (35.62±0.70 nmol/g) resulted in a significant inhibition of the ethanol induced increase of MDA (ANOVA F=5.084 P<0.05).
Effect of MCN on ethanol-induced changes in the levels of gastric mucosa myeloperoxidase (MPO) activity
Changes in gastric accumulation of neutrophils following ethanol induced gastric lesions were assessed by measurement of MPO activity. The effect of MCN on ethanol induced changes in the gastric mucosa MPO levels is given in Table 3. Administration of ethanol resulted in a significant increase in the levels of MPO (4.85±0.31 Δ/g tissue) as compared to the control animals (2.76±0.23 Δ/g tissue). Pretreatment with minocycline at 30 mg/kg (2.37±0.0.25 Δ/g tissue) and 100 mg/kg (2.70±0.028 Δ/g tissue) significantly attenuated ethanol induced increase in gastric MPO activity (ANOVA F=11.21 P<0.001). However the changes in the gastric MPO activity were not dose dependent and were statistically insignificant in rats pretreated with low dose (10 mg/Kg) of MCN (Table 3).
Discussion
The present investigation was undertaken to assess the gastro protective potential of minocycline against ethanol and indomethacin induced gastric ulceration in rats. The gastric mucosal protective activity of MCN was assessed by measuring gastric secretion, gastric adherent mucus, and markers of oxidative stress and neutrophil activation. The results of the present study suggest that minocycline has significant protective action against alcohol and indomethacin induced gastric ulcers. The results further indicate that the gastroprotective activity of MCN may be based on the regulation of gastric secretion and protection of gastric mucosa against the induced oxidative stress, and suppression of inflammation by inhibiting the activation of neutrophils.
Pretreatment of rats with MCN significantly reduced the gastric secretion and acidity in pylorus ligated shay rats (Table 1). Gastric acid plays a vital role in the development of gastric ulcer in pylorus ligated rats [28]. An enhanced acidity and gastric secretion are indicative of a disruption in the physiological mechanisms which normally regulate gastric secretion [37]. In addition it also results in the reduction of gastric pH, which leads to the activation of pepsinogen to pepsin. Pepsin by its proteolytic action further increases the size of the ulcer lesion [2]. Earlier studies have shown that agents with an ability to reduce gastric acid secretion help in the attenuation of gastric ulcers [13,15,38,39]. The gastro protective action of MCN may be assigned to the reduction in gastric secretion and acid output (Table 1).
Oral administration of ethanol resulted in the formation of ulcerative gastric lesions in glandular part of mucosa and a significant depletion in the levels of gastric wall mucus (Figures 1 & 2 and Table 2). Ulcerogenic substances such as aspirin, alcohol, non steroidal inflammatory substances (NSAIDs) etc., cause dispersal of the protective mucus gel and the phospholipids layer, resulting in acid back diffusion and mucosal injury [40,41]. Loss or depletion of gastro protective factors may also result in the breach of the gastric mucosal defense which may lead to ulceration even at the normal rate of acid secretion [42]. On the other hand, agents that restore the mucosal defense capacity exert a protective effect against ulcerogen induced gastrointestinal lesions [15,21,23,43]. Pretreatment with minocycline was not only helpful in the reduction of gastric secretion and acidity but also protected the gastric mucosa against ethanol induced gastric lesions and helped in restoring the depleted levels of gastric wall mucus (Figure 2 & Table 2). Increased secretion of mucus by gastric mucosal cells helps to prevent gastric ulcer by better buffering of the acidic gastric secretions, by reducing stomach wall friction during peristalsis and gastric contraction [44], and by assisting in the repair of the damaged gastric epithelium [45]. Epithelial cells of the gastric mucosa secrete mucus gel produced by mucin (MUC) genes [42]. Mucin and Zone Occludes-1 (Zone-1) a trans-membrane protein that preserves the tight junctions’ integrity are the indicators of mucosal epithelial integrity [46]. A decrease in their levels has been associated with mucosal damage in an animal model of inflammatory bowel disease (IBD) [23]. MCN was effective in augmenting the protective mechanism of the intestinal epithelial barrier and the restoration of the damaged histological structure in experimental IBD [23]. In addition, the beneficial effect of MCN was associated with an improvement of the altered immune response and reduction in the expression of the proinflammatory cytokines TNFα, IL1β and the chemokines MIP2 and MCP-1, the adhesion molecule ICAM1 and the enzyme INOS and matrix metalloproteinase, MMP9 [21,23] all known to play important roles in the mucosal damage [47]. Therefore, the cytoprotective effect of MCN against ethanol induced gastric lesions may be ascribed in part to its ability to strengthen the mucosal integrity by replenishing the depleted mucus layer (Table 2). This is further supported by the results of histological studies (Figure 3). MCN treated groups showed a better histological architecture with a more compact mucosa, less inflammation and hemorrhage (Figure 3C and 3D) which are in agreement with recent findings that show a protective effect of MCN in experimental models of intestinal mucositis and colitis [21-23].
We also observed MCN to wield a protective effect against indomethacin induced gastric lesions (Figure 4). Indomethacin induces gastric damage through various mechanisms including increased oxidative stress [13,48], suppression of prostaglandin generation, overproduction of leukotrienes [50], and enhanced neutrophil activity [49]. The gastro protective effect of MCN against indomethacin induced ulcers may be attributed to its ability, to scavenge free radicals and reduce oxidative stress [16,17,19,20], decrease pro-inflammatory cytokines [21], restore the depleted gastric mucus secretion (Table 2), inhibit enhanced MPO activity (Table 3) [50,51], and augmentation of prostaglandin E2 [52].
The precise mechanism of gastric ulceration is still not completely understood, but the gastric mucosal damage induced by ethanol has been suggested to be multifactorial that includes enhanced oxidative stress and depletion of antioxidants [13,15,53,54]. MDA is a marker for oxidative stress while sulfhydryls form the first line of defense against free radicals and help in cytoprotection against noxious stimuli [55]. A significant increase in the MDA levels following ethanol administration (Table 3) are in agreement with earlier studies [6,13,15,42,53,56] and suggest a massive release of reactive oxygen species (ROS). The ROS thus released may increase the MDA levels which in turn may damage the cell membrane [11]. The release of ROS has also been shown to play a vital role in ethanol induced gastric mucosal damage [10,57]. On the other hand, MCN is a potent anti-oxidant. The protective effect of MCN in animal models of neurotrauma and neurodegenerative diseases has been linked with decrease of oxidative and nitrosative stress and an increase in the level of antioxidants [17,19]. Its antioxidant ability is comparable to that of α tocopherol, and its water soluble analog Trolox [16] and is ten times more than that of doxycycline another tetracycline [51]. These properties are evident in the results of this study. Treatment of rats with minocycline resulted in a significant depletion of the ethanol induced MDA levels and restoration of the decreased NP-SH levels of the gastric tissue (Table 3). Nonprotein sulfhydryls are endogenous anti-oxidants and protect the cells by different mechanisms, primarily by limiting the production of free radicals [58,59]. Secondarily, they protect the mucous by strengthening the disulfide bridges of its subunits. A decrease in these bridges reduces the mucus thickness and makes it more vulnerable to harmful agents [60]. Changes in the SH levels may be crucial for ulcer formation, given that they help in the preservation of mucosal integrity, and their reduced levels in the gastric mucosa result in the formation of gastric ulcers [61]. Moreover, depletion in glutathione levels results in a significant increase of ulcerogens induced gastric mucosal damage [1], while compounds that augment the mucosal NP-SH levels have gastro protective effects [13,14,39,40]. Therefore, the cytoprotective and anti-ulcerogenic effects of MCN may be ascribed to the direct antioxidant and free radical scavenging activity of MCN and also to the augmentation of the NP-SH levels (Table 3).
Besides generation of free radicals, infiltration of inflammatory cells (neutrophils, macrophages) also plays a major role in ethanol induced gastric ulcers [5]. Accumulation of neutrophils may lead to microcirculatory disturbances and induce ischemia by occluding microvessels [62]. Activated neutrophils also release many injurious factors that include free radicals, metabolites and proteolytic enzymes [63-67]. MPO, which is expressed in neutrophils, is considered as a reliable indicator for neutrophil infiltration and inflammation in the gastric mucosa [7,9,13-15]. We observed a significant increase in gastric mucosal MPO activity after treatment with ethanol indicating an increase in the infiltration of activated neutrophils. On the other hand, treatment with MCN had a highly attenuating effect on the ethanol induced increase in MPO activity (Table 3). Minocycline and doxycycline another tetracycline has been shown to significantly decrease the infiltration of neutrophils and MPO release and exert cytoprotective effects [51,52]. A decrease in the MPO activity along with the reduction in the gastric lesions in MCN pretreated rats suggest that the anti-ulcerogenic effect of MCN may also be due to its anti-inflammatory and inhibitory action on neutrophil infiltration.
In conclusion, the results of the present study suggest that MCN has significant gastro protective effects against both ethanol and indomethacin induced gastric ulcers. The protective effects against ethanol induced gastric ulcers were associated with reduced gastric secretion, oxidative stress, neutrophil activity and gastric mucosal breakdown. However further detailed studies are warranted for the use of MCN as a therapy for gastric ulcers.
Acknowledgements
The authors are thankful to the Prince Sultan Military Medical City and the Medical Services Department, Ministry of Defense for their encouragement and support.
Disclosure of conflict of interest
None.
References
- 1.Saxena B, Krishnamurthy S, Singh S. Gastroprotective potential of risperidone, an atypical antipsychotic, against stress and pyloric ligation induced gastric lesions. Chem Biol Interact. 2011;190:155–164. doi: 10.1016/j.cbi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Verma M. A review of peptic ulcer: A Global threat. J Pharm Res. 2010;3:2088–2091. [Google Scholar]
- 3.Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L. ) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156–63. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2011;88:1547–65. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 5.Alican I, Coskun T, Corak A, Yegen BC, Oktay S, Kurtel H. Role of neutrophils in indomethacin-induced gastric mucosal lesions in rats. Inflamm Res. 1995;44:164–168. doi: 10.1007/BF01782814. [DOI] [PubMed] [Google Scholar]
- 6.Alimi H, Faiedh N, Bouoni Z, Hfaiedh M, Sakly M, Zourgui L. Antioxidant and antiulcerogenic activities of Opuntia ficus f. inermis root extract in rats. Phytomedicine. 2010;17:1120–1126. doi: 10.1016/j.phymed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Tuluce Y, Ozkol H, Koyuncu I, Ine H. Gastroprotective effect of small centaury (Centaurium erythraea L) on aspirin-induced gastric damage in rats. Toxicol Ind Health. 2011;27:760–768. doi: 10.1177/0748233710397421. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Tian X, Gou L, Fu X, Li S, Lan N, Yin X. Protective effect of L-citrulline against ethanol-induced gastric ulcer in rats. Environ Toxicol Pharmacol. 2012;34:280–287. doi: 10.1016/j.etap.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Lee MY, Shin IS, Jeon WY, Seo CS, Ha H, Huh JI, Shin HK. Protective effect of Bojungikki-tang, a traditional herbal formula, against alcohol-induced gastric injury in rats. J Ethnopharmacol. 2012;142:346–353. doi: 10.1016/j.jep.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Smith GS, Mercer DW, Cross JM, Barreto JC, Miller TA. Gastric injury induced by ethanol and ischemia-reperfusion in the rat. Dig Dis Sci. 1996;41:1157–1164. doi: 10.1007/BF02088232. [DOI] [PubMed] [Google Scholar]
- 11.Tariq M, Khan HA, Elfaki I, Arshaduddin M, Al Moutaery M, Al Rayes H, Al Swailam R. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem Biol Interact. 2007;170:40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 13.Tariq M, Khan HA, Elfaki I, Arshaduddin M, Al Moutaery M, Al Rayes H, Al Swailam R. Gastric antisecretory and antiulcer effects of simvastatin in rats. J Gastroenterol Hepatol. 2007;22:2316–23. doi: 10.1111/j.1440-1746.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- 14.Al Asmari AK. Al Omani S, Elfaki I, Tariq M, Al Malki A, Al Asmary S. Gastric antisecretory and anti ulcer activity of bovine hemoglobin. World J Gastroenterol. 2013;21:3291–3299. doi: 10.3748/wjg.v19.i21.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Moutaery M, Al Rayes H, Al Swailam R, Elfaki I, Arshaduddin M, Tariq M. Protective effect of a cysteine prodrug and antioxidant, L-2-oxothiazolidine-4-carboxylate, against ethanol-induced gastric lesions in rats. Exp Toxicol Pathol. 2012;64:233–237. doi: 10.1016/j.etp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–827. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- 17.Schildknecht S, Pape R, Müller N, Robotta M, Marquardt A, Bürkle A, Drescher M, Leist M. Neuroprotection by minocycline cause by direct and specific scavenging of peroxynitrite. J Biol Chem. 2011;286:4991–5002. doi: 10.1074/jbc.M110.169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–8. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padi SS, Kulkarni SK. Minocycline prevents the development of neurophatic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol. 2008;601:79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Singh LP, Mishra A, Saha D, Swarnakar S. Doxycycline blocks gastric ulcer by regulating matrix metalloproteinase-2 activity and oxidative stress. World J Gastroenterol. 2011;17:3310–3321. doi: 10.3748/wjg.v17.i28.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang TY, Chu HC, Lin YL, Ho WH, Hou HS, Chao YC, Liao CL. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009;389:634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, Chao YC, Liao CL. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinase. Toxicol Appl Pharmacol. 2009;237:69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, Rodríguez-Cabezas ME, Gálvez J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol. 2011;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailón E, Cueto-Sola M, Utrilla P, Nieto A, Zarzuelo A, Rodríguez-Cabezas ME, Gálvez J. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011;63:308–19. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris pharmacokinetic and pharmacodynamic perspectives. J Clin Aesthet Dermatol. 2011;4:40–47. [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K, Sato R, Okimoto T, Watanabe K. Effectiveness of minocycline-based triple therapy for eradication of Helicobater. J Gastroenterol Hepatol. 2006;21:262–7. doi: 10.1111/j.1440-1746.2006.04183.x. [DOI] [PubMed] [Google Scholar]
- 27.Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, Fukuda K, Katsurahara M, Ito T, Cesar GE, Imoto I, Takei Y. Annual change of primary resistance to clarithromycin among Helicobter pylori isolate from 1996 through 2008 in Japan. Helicobacter. 2009;14:86–90. doi: 10.1111/j.1523-5378.2009.00714.x. doi: 10.111/j. 523-5378.2009.0714.x. [DOI] [PubMed] [Google Scholar]
- 28.Shay H, Kumarov SA, Fels SA, Meraze D, Gruenstein M, Siplet H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 29.Bhargava KP, Gupta MB, Tangri KK. Mechanism of ulcerogenic activity of indomethacin and ocyphenbutazone. Eur J Pharmacol. 1973;22:191–5. doi: 10.1016/0014-2999(73)90012-5. [DOI] [PubMed] [Google Scholar]
- 30.Valcavi U, Caponi R, Brambilla A. Gastric antisecretory, antiulcer and cytoprotective properties of 9-hydroxy-19, 20-bis-norprostanoid acid in experimental animals. Arzneimittelforschung. 1982;32:657–63. [PubMed] [Google Scholar]
- 31.Natale G, Lazzeri G, Blandizzi C, Gherardi G, Lenzi P, Pellegrini A, Del Tacca M. Seriate histomorphometry of whole rat stomach: an accurate and reliable method of quantitative analysis of mucosal damage. Toxicol Appl Pharmacol. 2001;174:17–26. doi: 10.1006/taap.2001.9193. [DOI] [PubMed] [Google Scholar]
- 32.Schianterelli P, Cadel S, Folco GS. Gastroprotective effects of morniflumate, an esterified anti-inflammatory drug. Arzneimittelforschung. 1984;34:885–890. [PubMed] [Google Scholar]
- 33.Corne SJ, Morrisey SM, Woods RJ. A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116–17. [PubMed] [Google Scholar]
- 34.Sedlak J, Lindsay RH. Estimation of total protein bound and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–5. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 35.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 36.Bradley PP, Erdman SH, Christensen RD, Rothstein G. Supply and release of storage neutrophils, a developmental study. Biol Neonate. 1984;41:132–7. doi: 10.1159/000241541. [DOI] [PubMed] [Google Scholar]
- 37.Goa KL, Monk JP. Enprostil: a preliminary review of its pharmacodynamics and pharmacokinetic properties and therapeutic efficacy in the treatment of peptic ulcer disease. Drugs. 1987;3:539–59. doi: 10.2165/00003495-198734050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Schubert LM, Puera DA. Control of acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastro protective effect of cyaniding 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42:683–687. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Alqasoumi S, Al-Howiriny TA, Al-Yahya M, Rafatulla S. Gastroprotective effects of radish “raphanus sativus” L. on experimental gastric ulcer models in rats. Farmacia. 2008;56:204–214. [Google Scholar]
- 41.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 42.Laine L, Weinstein WM. Histology of alcoholic haemorrhagic gastritis; a prospective evaluation. Gastroenterology. 2008;94:1254–64. doi: 10.1016/0016-5085(88)90661-0. [DOI] [PubMed] [Google Scholar]
- 43.Sannomiya M, Fonseca VB, da Silva MA, Rocha LR, Dos Santos LC, Hiruma-Lima CA, Souza Brito AR, Vilegas W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J Ethnopharmacol. 2005;97:1–6. doi: 10.1016/j.jep.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 44.Alqasoumi S, Al-Sohaibani M, Al-Howiriny T, Al-Yahya M, Rafatullah S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J Gastroenterol. 2009;15:1958–65. doi: 10.3748/wjg.15.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripathi KD. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2008. p. 597. [Google Scholar]
- 46.Wallace JL, Whittle BJ. Role of mucus in the repair of gastric epithelial damage in the rat. Inhibition of epithelial recovery by mucolytic agents. Gastroenterology. 1986;91:603–611. doi: 10.1016/0016-5085(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 47.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–9. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otamiri T, Tagesson C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am J Surg. 1989;157:562–5. doi: 10.1016/0002-9610(89)90699-5. [DOI] [PubMed] [Google Scholar]
- 50.Wallace JL. Gastric ulceration: critical events at the neutrophil-endothelium interface. Can J Physiol Pharmacol. 1992;71:98–102. doi: 10.1139/y93-014. [DOI] [PubMed] [Google Scholar]
- 51.Leite LM, Carvalho AG, Ferreira PL, Pessoa IX, Gonçalves DO, Lopes Ade A, Góes JG, Alves VC, Leal LK, Brito GA, Viana GS. Anti-inflammatory properties of doxycycline and minocycline in experimental models: an in vivo and in vitro comparative study. Inflammopharmacology. 2011;19:99–110. doi: 10.1007/s10787-011-0077-5. [DOI] [PubMed] [Google Scholar]
- 52.Huang C, Li R, Zeng Q, Ding Y, Zou Y, Mao X, Hu W, Xiong R, Li M. Effect of minocycline postconditioning and ischemic postconditioning on myocardial ischemia-reperfusion injury in atherosclerosis rabbits. J Huazhong Univ Sci Technolog Med Sci. 2012;32:524–9. doi: 10.1007/s11596-012-0090-y. [DOI] [PubMed] [Google Scholar]
- 53.Patel RN, Attur MG, Dave MN, Patel VI, Stuchin SA, Abramson SA, Amin AR. A novel mechanism of action of chemically modified tetracycline: Inhibition off COX2 mediated prostaglandin E2 production. J Immunol. 1999;163:3459–3467. [PubMed] [Google Scholar]
- 54.Wang Y, Su W, Zhang C, Xue C, Chang Y, Wu X, Tang Q, Wang J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chemistry. 2012;133:1414–1419. [Google Scholar]
- 55.Ihtiyar E, Yaşar NF, Erkasap N, Köken T, Tosun M, Oner S, Erkasap S. Effects of doxycycline on intestinal ischemia reperfusion injury induced by abdominal compartment syndrome in a rat model. Curr Ther Res Clin Exp. 2010;71:186–198. doi: 10.1016/j.curtheres.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 57.Santos CG, dos Santos SG, Rios VE, Fragoso de Freitas AP, Arcanjo MB, Silveira MD, Lopes SA, Barbosa Filho JM, de Almeida Leal LD, de Castro Brito GA, Souccar C, de Barros Viana GS. Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur J Pharmacol. 2012;683:260–9. doi: 10.1016/j.ejphar.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 58.Pihan C, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- 59.Wakulich C, Tepperman BL. Role of glutathione in nitric oxide-mediated injury to rat gastric mucosal cells. Eur J Pharmacol. 1997;319:333–341. doi: 10.1016/s0014-2999(96)00865-5. [DOI] [PubMed] [Google Scholar]
- 60.La Casa C, Villegas I, Alarcon de la Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, natural flavones, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 61.Avila C, Paul VJ. Chemical ecology of the nudibranch Glossodoris pallid: is the location of diet-derived metabolites important for defense? Mar Ecol Prog Ser. 1996;150:171–180. [Google Scholar]
- 62.Hoppenkamps R, Thies E, Younes M, Siegers CP. Glutathione and GSH dependent enzymes in the human gastric mucosa. Klin Wochenschr. 1984;62:183–186. doi: 10.1007/BF01731642. [DOI] [PubMed] [Google Scholar]
- 63.Kitahora T, Guth PH. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987;93:810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- 64.Reiter RJ, Gangluly K, Kundu P, Banerjee A, Swarnakar S. Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic Biol Med. 2006;41:911–925. doi: 10.1016/j.freeradbiomed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan G, Maguire D, Collins JK, Morgan J, Shanahan F. Bone marrow Micrometastases and gastrointestinal cancer detection and significance. Am J Gastroenterol. 2000;95:1644–1651. doi: 10.1111/j.1572-0241.2000.02199.x. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi T, Ohta Y, Yoshino J, Nakazawa S. Teprenone promotes the healing of acetic acid-induced chronic gastric ulcers in rats by inhibiting neutrophil infiltration and lipid peroxidation in ulcerated gastric tissues. Pharmacol Res. 2001;43:23–30. doi: 10.1006/phrs.2000.0748. [DOI] [PubMed] [Google Scholar]
- 67.Alimi H, Hfaiedh N, Bouoni Z, Sakly M, Ben Rhouma K. Evaluation of antioxidant and antiulcerogenic activities of Opuntia ficus indica f. inermis flowers extract in rats. Environ Toxicol Pharmacol. 2011;32:406–16. doi: 10.1016/j.etap.2011.08.007. [DOI] [PubMed] [Google Scholar]


