Abstract
Objective: A group of healthy females were randomly sampled in Shanghai for the purpose of evaluating the prevalence of urinary incontinence (UI) among Chinese women and its associated risk factors. Methods: 5,467 registered female residents aged from 20 years were randomly sampled from four communities in Shanghai, and the Bristol Female Lower Urinary Tract Symptoms Questionnaire (the International Consultation Incontinence Questionnaire) was adopted. Detailed information regarding pregnancy, menstruation, and several demographic variables was also collected. Data were subsequently analyzed using McNemar’s test, univariate analysis, multinomial logistic regression models, and binary logistic regression models. Results: The prevalence rate of UI was estimated to be 23.3%. The rate of stress UI (SUI) was 14.0% (761/5433), which was more prevalent than the rate of urgency UI (UUI) and mixed UI (MUI), which were 3.0% (164/5433) and 6.3% (341/5433), respectively. The risk factors associated with developing UI included aging, lack of education, poor living environment (specifically in the rural area), intense manual labor, the absence of physical exercise, hyperlipemia, diabetes, nocturia, consumption of greasy food, divorce or widowing, chronic pelvic pain (CPP), pelvic organ prolapse (POP), frequent urinary tract infection, and vaginal delivery without episiotomy. We also observed that most Chinese women were not aware of UI, which prevented them from seeking early treatment. Conclusions: Urinary incontinence is a common disorder among Chinese women in Shanghai, and many risk factors contribute to the development of UI. Most Chinese women were not aware of UI, which prevented them from seeking early treatment.
Keywords: Urinary incontinence, prevalence, risk factors, Chinese women
Introduction
According to many epidemiological studies, more than 40% of women all over the world are suffering from urinary incontinence (UI) physically, psychologically, socially, and economically [1-3]. UI has become a global problem for women, as well as a heavy financial burden on the health-care systems of many countries. As the Chinese population ages, UI among women begins to draw more attention in China. However, common unawareness and the lack of knowledge regarding UI make it difficult for physicians to perform early diagnosis and treatment. The current high prevalence rate of UI in China calls for urgent prevention and control strategies for UI. Successful formulation of such strategies can reduce the financial cost associated with treating this disorder and provide valuable experiences for other countries. There have been some studies on the prevalence rates of UI in many regions of China, but their results vary greatly. This variation might be attributed to differences in factors such as survey method, level of education of participants, sample size, definition of UI used in the study, and duration of the study. Shanghai is one of the most representative modern metropolises but has yet to make any reported prevalence rate and risk factors of UI available to the public. Therefore, we conducted a population-based survey in Shanghai between 2010 and 2012 to estimate the current prevalence rate of UI and to identify its associated risk factors among Chinese women in Shanghai.
Subjects and methods
Participants and design of the survey
Between March 2010 and September 2012, we conducted a survey in Shanghai, China. We first assumed the prevalence rate of UI to be 10%, which was close to the lowest rate across the world. According to the formula: Sufficient sample size = 400 × (1 - Proportion) / Proportion, a sample consisting of more than 3,600 participants would be sufficient for this survey. In this survey, a multiple-stage stratified cluster systematic sampling technique was applied. In the first stage of selection, two urban districts and two rural districts from the city’s ten urban districts and ten rural ones were randomly selected. Then one community from each of the selected districts was then randomly selected. Finally, we chose 1,000 households from the community above and surveyed all female members in these households. Eligible female participants had to meet the following key criteria: 1) 20 years old and older (legal age of marriage for women in China), 2) permanent resident of Shanghai, 3) have clear consciousness, and 4) provide informed consent.
All the participants were surveyed face-to-face by a team of researchers, which consisted of three urologists and four assistants from local communities. The researchers used a standardized questionnaire adapted from the International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms Long Form. The questionnaire collects the participants’ demographic characteristics (age, residence, occupation, education background, marital status, BMI, income, etc.), behavioral data (smoking, alcohol consumption, level of physical exercise, etc.), history of chronic diseases, gynecologic and obstetric information (age of menarche, menstrual status, history of pregnancy and abortion, mode of delivery, birth weight of the baby, history of pelvic surgery, history of gynecologic disease, etc.), defecation data, and other information defining UI, subtypes of UI (SUI, UUI, and MUI), OABSS or severity of SUI and nocturia.
A pilot study consisting of approximately 100 participants was performed in advance to assess the questionnaire’s reliability and validity. The results of the pilot study indicated good reliability, with a Cronbach’s alpha coefficient of 0.82 and Guttman’s split-half reliability value of 91. The survey content was approved by the Institutional Review Board and Human Research Ethics Committee of the Shanghai Center for Disease Prevention and Control.
Definitions and categories
According to the International Continence Society [4], UI is defined as the complaint of any involuntary loss of urine and is categorized into the following subtypes: 1) Stress urinary incontinence (SUI) is the complaint of involuntary leakage of urine upon effort, exertion, sneezing, or coughing. 2) Urgency urinary incontinence (UUI) is the complaint of involuntary leakage of urine accompanied by or immediately preceded by urgency. 3) Mixed urinary incontinence (MUI) is the complaint of involuntary leakage of urine associated with urgency and also with effort, exertion, sneezing, and coughing. We assigned one of the three subtypes of UI to each participant reporting positive symptoms, based on her answers in the questionnaire.
Other definitions and categories are as follows:
1) Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Our researchers calculated BMI for each participant using their reported height and weight. The subjects were grouped by BMI into three subcategories: less than or equal to 25, between 25 and 28, and greater than 28.
2) Two categories for monthly income were used: less than RMB 2,500 and greater than RMB 2,500. The amount of RMB 2,500 per month was used because it was the cut-point for receiving low-income welfare.
3) Education level included two categories: voluntary education (high school and below) and above voluntary education (college and above).
4) We divided all participants into two groups according to the intensity of manual labor: manual laborer and non-manual laborer, using definitions from the Standard of the National Manual Labor Strength Grade Manual.
4) Two categories for residence were used: urban and rural.
5) Marital status included three categories: divorced or widowed, unmarried, and married.
6) Frequency of smoking included two categories: smoke often (one or more cigarettes on average per day) and smoke occasionally (less than one cigarette on average per day).
7) Frequency of alcohol consumption was categorized as follows: drink often (drink once or more often on average per day) and drink occasionally (drink less than once on average per day).
8) Level of physical exercise was categorized into more than or equal to eight times per month and less than times per month. Eight sessions of physical exercise per month is the optimal amount, according to expert opinion [5].
9) Age of menopause was categorized into N/A (regular condition in menstrual cycle and flow), over 45, and 45 or before.
10) The history of disease category examined whether the participant was diagnosed with each of the following conditions: respiratory diseases, nocturia, chronic pelvic pain (CPP), pelvic organ prolapse, (POP), urinary tract infection cardiovascular disease, hyperlipemia, constipation, and diabetes.
11) Mode of delivery included four categories: nullipara, cesarean section, vaginal delivery with episiotomy (including medio-lateral episiotomy and lateral episiotomy), and vaginal delivery without episiotomy.
Statistical analysis
Data collected from questionnaires was entered in duplicate using EpiData software and analyzed using the SPSS 16.0 statistical package (IBM SPSS, Chicago, IL). Median and range values were calculated for continuous variables, and percentages were calculated for categorical variables. The prevalence rates of UI and its subtypes were compared between subgroups using the χ2 test. Univariate and multivariate unconditional logistic regression analysis were conducted to determine the risk factors associated with UI in participants. The variables with P < 0.20 in univariate analysis were included in the multivariate analysis. Backward logistic regression was conducted by removing variables with P > 0.10. Statistical significance was defined as P < 0.05.
Results
Demographic characteristics of participants
A total of 5,467 women were selected to participate in the survey, and 99.1% (5433/5467) completed the survey. The age of all participants ranged from 20 to 100, and the median was 46.8 years. Approximately 50.4% (2642/ 5433) lived in urban areas. More than half of the participants (77.3%, 4201/5433) were non-manual laborers. In addition, 65.9% (3579/5433) completed voluntary education. Approximately 81.3% (4418/5433) were married or remarried. The proportion of overweight participants (with BMI greater than 25) was 20.6% (1118/5433). In addition, 47.7% (2591/5433) of the participants reported monthly income of over RMB 2,500. Approximately 2.3% (124/5433) smoked often, and 1.0% (55/5433) drank often. Approximately 26.3% (1430/5433) were able to perform physical exercise more than eight times per month. The detailed demographic characteristics of the participants are presented in Table 1.
Table 1.
Demographic Characteristics of Participants
| Variable | Group | Value (%) |
|---|---|---|
| Age | 20-39 | 2341 (43.1) |
| 40-49 | 854 (15.7) | |
| 50-59 | 821 (11.6) | |
| 60-69 | 632 (11.6) | |
| 70-79 | 468 (8.6) | |
| 80-100 | 317 (5.8) | |
| BMI | > 28 | 143 (2.6) |
| 25-28 | 975 (17.9) | |
| < 25 | 4315 (79.4) | |
| Monthly Income | < 2500 | 2842 (52.3) |
| > 2500 | 2591 (47.7) | |
| Education | Voluntary education | 1854 (34.1) |
| Above voluntary education | 3579 (65.9) | |
| Residence | Urban area | 2753 (50.7) |
| Rural area | 2680 (49.3) | |
| Labor | Non-manual laborer | 4201 (77.3) |
| Manual laborer | 1232 (22.7) | |
| Physical Exercise | < 8 times per month | 4003 (73.7) |
| ≥ 8 times per month | 1430 (26.3) | |
| Hyperlipemia | No | 4790 (88.2) |
| Yes | 643 (11.8) | |
| Heart disease | No | 5353 (98.5) |
| Yes | 80 (1.5) | |
| Nervous System Disease | No | 5338 (98.3) |
| Yes | 95 (1.7) | |
| Diabetes | No | 5174 (95.2) |
| Yes | 259 (4.8) | |
| Nocturia | < 2 | 4275 (78.7) |
| > 2 | 1137 (20.9) | |
| Constipation | No | 3858 (71.0) |
| Yes | 1575 (29.0) | |
| Alcohol Consumption | Less than once per day | 5309 (97.7) |
| More than once per day | 124 (2.3) | |
| Smoking | More than one day | 5378 (99.0) |
| Less than one per day | 55 (1.0) | |
| Greasy Food Consumption | Everyday | 924 (17.0) |
| > 3 times per week | 1888 (34.8) | |
| > 1 times per week | 1539 (28.3) | |
| < 1 times per week | 1082 (19.9) | |
| Age of Menopause | N/A | 3551 (65.4) |
| < 45 | 236 (4.3) | |
| > 45 | 1646 (30.3) | |
| POP | No | 5387 (99.2) |
| Yes | 46 (0.8) | |
| Chronic Pelvic Pain | No | 5175 (95.3) |
| Yes | 258 (4.7) | |
| Marital Status | Divorced or Widowed | 456 (8.4) |
| Unmarried | 559 (10.3) | |
| Married | 4418 (81.3) | |
| Prolonged Labor | No | 5334 (98.2) |
| Yes | 99 (1.8) | |
| Respiratory Disease | No | 4995 (91.9) |
| Yes | 438 (8.1) | |
| Pregnancy | Vaginal delivery > 1 | 1001 (18.4) |
| Vaginal delivery = 1 | 2569 (47.3) | |
| N/A | 1893 (34.3) | |
| Urinary Tract Infection Frequency | No | 5321 (97.9) |
| Yes | 112 (2.1) | |
| Mode of Delivery | Nullipara | 761 (14.0) |
| Cesarean section | 1132 (20.8) | |
| Vaginal delivery with episiotomy | 1918 (35.3) | |
| Vaginal delivery without episiotomy | 1622 (29.9) | |
| In Treatment | No | 5164 (95.0) |
| Yes | 269 (5.0) |
Prevalence rates of UI
The prevalence rate of UI was estimated to be 23.3%. The rate of stress UI (SUI) was 14.0% (761/5433), which was more prevalent than the rate of urgency UI (UUI) and mixed UI (MUI), which were 3.0% (164/5433) and 6.3% (341/5433), respectively. The prevalence rates of all SUI, UUI, and MUI all increased with age and peaked between the age of 80 and 100 years. A sharp rise was observed between 40 and 49 years old, when the rate of SUI increased dramatically. In addition, there was also a sharp rise between the ages of 70 to 79 years, mostly because of an increasing risk of MUI (Figure 1, Table 2).
Figure 1.
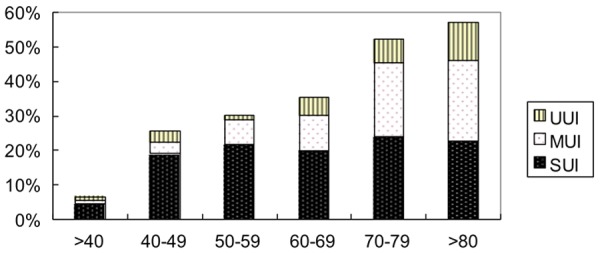
Prevalence of UI by Age. The prevalence rates of all SUI, UUI, and MUI all increased with age. A sharp rise was observed between 40 and 49 years old, when the rate of SUI increased dramatically. In addition, there was also a sharp rise between the ages of 70 to 79 years, mostly because of an increasing risk of MUI.
Table 2.
Prevalence of Urinary Incontinence by Age
| Age Group | Types of UI (%) | Total | |||
|---|---|---|---|---|---|
|
| |||||
| Not Infected | SUI | MUI | UUI | ||
| 20-39 | 2187 (93.4) | 109 (4.7) | 16 (0.7) | 29 (1.2) | 2341 |
| 40-49 | 637 (74.6) | 162 (19.0) | 28 (3.3) | 27 (3.2) | 854 |
| 50-59 | 574 (69.9) | 179 (21.8) | 59 (7.2) | 9 (1.1) | 821 |
| 60-69 | 409 (64.7) | 126 (19.9) | 65 (10.3) | 32 (5.1) | 632 |
| 70-79 | 224 (47.9) | 113 (24.1) | 99 (21.2) | 32 (6.8) | 468 |
| 80-100 | 136 (42.9) | 72 (22.7) | 74 (23.3) | 35 (11.0) | 317 |
| Total | 4167 (76.7) | 761 (14.0) | 341 (6.3) | 164 (3.0) | 5433 |
Factors associated with urinary incontinence
In the univariate analysis, we discovered a number of significant factors associated with UI, including age, overweight, income, level of education, residence, intensity of physical labor, frequency of physical exercise, hyperlipemia, heart disease, nervous system disease, diabetes, times of nocturia, constipation, alcohol consumption, smoking, consumption of greasy food, age of menopause, pelvic organ prolapse, chronic pelvic pain, marital status, prolonged labor, respiratory disease, multiple pregnancy, urinary tract infection frequency, and mode of delivery. More details can be found in Table 3.
Table 3.
Univariate Analysis of Risk Factors Associated with Urinary Incontinence
| Factories | UI case (%) | Control (%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Overweight | BMI > 28 | 57 (4.5) | 86 (2.1) | 2.22 (1.56-3.13) | < 0.01 |
| BMI 25-28 | 218 (17.2) | 757 (18.2) | 0.97 (0.82-1.14) | 0.672 | |
| BMI < 25 | 992 (78.3) | 3323 (79.8) | |||
| Income | < 2500 | 884 (69.8) | 1958 (47) | 0.38 (0.33-0.44) | < 0.01 |
| > 2500 | 383 (30.2) | 2208 (53.0) | |||
| Education | Voluntary education | 588 (46.6) | 1261 (30.3) | 0.52 (0.45-0.59) | < 0.01 |
| Above voluntary education | 672 (53.3) | 2904 (69.7) | |||
| Residence | Urban area | 569 (44.9) | 2184 (52.4) | 1.35 (1.19-1.53) | < 0.01 |
| Rural area | 698 (55.1) | 1982 (47.6) | |||
| Labor | Non-manual labor | 804 (63.5) | 3397 (81.5) | 2.54 (2.22-2.92) | < 0.01 |
| Manual labor | 463 (36.5) | 769 (18.5) | |||
| Physical exercise | < 8 times per month | 883 (69.7) | 3120 (74.9) | 1.30 (1.13-1.49) | < 0.01 |
| ≥ 8 times per month | 384 (30.3) | 1046 (25.1) | |||
| Hyperlipemia | No | 930 (73.4) | 3860 (92.7) | 4.57 (3.85-5.42) | < 0.01 |
| Yes | 337 (26.6) | 306 (7.3) | |||
| Heart disease | No | 1208 (95.3) | 4145 (99.7) | 9.64 (5.83-15.9) | < 0.01 |
| Yes | 59 (4.7) | 21 (0.3) | |||
| Nervous system disease | No | 1224 (96.6) | 4114 (98.8) | 2.78 (1.85-4.18) | < 0.01 |
| Yes | 43 (3.4) | 52 (1.2) | |||
| Diabetes | No | 1114 (87.9) | 4060 (97.5) | 5.26 (4.07-6.80) | < 0.01 |
| Yes | 153 (12.1) | 106 (2.5) | |||
| Times of Nocturia | < 2 | 601 (47.5) | 3674 (88.6) | 8.61 (7.44-9.97) | < 0.01 |
| > 2 | 665 (52.5) | 472 (11.4) | |||
| Constipation | No | 740 (58.4) | 3118 (74.8) | 2.119 (1.86-2.42) | < 0.01 |
| Yes | 527 (41.6) | 1048 (25.2) | |||
| Alcohol drinking | < 2 times/week | 1228 (96.9) | 4081 (98.0) | 2.93 (1.038-2.24) | 0.03 |
| > 2 times/week | 39 (3.1) | 85 (2.00) | |||
| Smoking Groups | < 40 times/week | 1247 (98.4) | 4131 (99.2) | 1.89 (1.09-3.29) | 0.021 |
| 20-39 | 20 (1.6) | 35 (0.8) | |||
| Greasy Food eating | Everyday | 176 (13.9) | 748 (18) | 1.18 (1.11-1.26) | < 0.01 |
| > 3 times/week | 417 (32.9) | 1471 (35.3) | |||
| > 1 times/week | 363 (28.7) | 1176 (28.2) | |||
| < 1 times/week | 311 (24.5) | 771 (18.5) | |||
| Age of c | No | 448 (35.4) | 3103 (74.5) | 0.19 (0.16-0.22) | < 0.01 |
| < 45 | 102 (8.1) | 134 (3.2) | 0.97 (0.75-1.30) | 0.92 | |
| > 45 | 717 (56.6) | 929 (22.3) | |||
| Pelvic organ prolapse | No | 1234 (97.4) | 4153 (99.7) | 8.54 (4.48-16.28) | < 0.01 |
| Yes | 33 (1.3) | 13 (0.3) | |||
| Chronic pelvic pain | No | 1142 (90.1) | 4033 (96.8) | 3.32 (2.58-4.28) | < 0.01 |
| Yes | 125 (9.9) | 133 (3.2) | |||
| Marital status | Divorced or Widowed | 197 (15.5) | 259 (6.2) | 2.39 (1.96-2.91) | < 0.01 |
| Unmarried | 3 (0.2) | 556 (13.3) | 0.017 (.005-0.53) | < 0.01 | |
| Married | 1067 (84.2) | 3351 (80.4) | |||
| Prolonged labor | No | 1195 (94.7) | 4134 (99.2) | 7.78 (5.11-11.86) | < 0.01 |
| Yes | 72 (5.3) | 32 (5.3) | |||
| Respiratory disease | No | 1081 (85.3) | 3914 (94.0) | 2.67 (2.19-3.27) | < 0.01 |
| Yes | 186 (14.7) | 252 (6.0) | |||
| Pregnancy | > 1 | 492 | 503 | 8.31 (6.86-10.06) | < 0.01 |
| = 1 | 503 | 1947 | 2.50 (2.10-2.97) | ||
| Else | 202 | 1716 | |||
| Urinary tract infection | No | 1196 (94.4) | 4128 (99.0) | 3.62 (2.91-4.50) | < 0.01 |
| Yes | 71 (5.6) | 40 (1.0) | |||
| Mode of delivery | Nullipara | 13 (1.0) | 748 (18.0) | 0.024 (0.014-.042) | < 0.01 |
| Cesarean section | 134 (10.6) | 998 (24.0) | 0.19 (0.15-0.23) | < 0.01 | |
| Vaginal delivery with episiotomy | 443 (35.0) | 1475 (35.4) | 0.42 (0.36-0.49) | < 0.01 | |
| Vaginal delivery without episiotomy | 677 (53.4) | 945 (22.7) | |||
Most character variables included in the univariate logistic regression model are listed in Table 4. Multivariate analysis indicated that older age (OR = 2.38, 95% CI 2.15-2.62, P < 0.01), lower education (OR = 2.83, 95% CI 2.31-3.47, P < 0.01), living in a rural area (OR = 1.94, 95% CI 1.64-2.30, P < 0.01), manual labor worker (OR = 6.90, 95% CI 5.64-8.44, P < 0.01), lacking physical exercise (OR = 0.56, 95% CI 0.47-0.68, P < 0.01), hyperlipemia (OR = 1.99, 95% CI 1.57-2.53, P < 0.01), diabetes (OR = 1.68, 95% CI 1.19-2.36, P = 0.003), nocturia (OR = 8.24, 95% CI 6.75-10.05, P < 0.01), constipation (OR = 7.45, 95% CI 5.95-9.32, P < 0.01), greasy food consumption (OR = 0.80, 95% CI 0.73-0.88, P < 0.01), and divorce or widowing (OR = 2.06, 95% CI 1.58-2.69, P < 0.01) are associated with the risk of developing UI. In addition, unmarried women display a lower risk compared with the married (OR = 0.14, 95% CI 0.04-0.43, P = 0.001). Constipation, manual labor, and times of nocturia are also associated with the development of UI. Some other factors, such as BMI, income, heart disease, nervous system disease, alcohol consumption, and smoking were not associated with the development of UI.
Table 4.
Multivariate Analysis of Factors Associated With Urinary Incontinence
| Factories | UI case (%) | Control (%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Age in years, median | 20-39 | 154 (12.2) | 2187 (52.5) | 2.38 (2.16-2.63) | < 0.01 |
| 40-49 | 217 (17.1) | 637 (15.3) | |||
| 50-59 | 247 (19.5) | 574 (13.8) | |||
| 60-69 | 223 (17.6) | 409 (9.8) | |||
| 70-79 | 244 (19.3) | 224 (5.4) | |||
| 80-100 | 136 (14.4) | 135 (3.2) | |||
| Overweight | BMI > 28 | 57 (4.5) | 86 (2.1) | 1.12 (0.98-1.36) | 0.08 |
| BMI 25-28 | 218 (17.2) | 757 (18.2) | |||
| BMI < 25 | 992 (78.3) | 3323 (79.8) | |||
| Income | < 2500 | 884 (69.8) | 1958 (47) | 1.02 (0.79-1.32) | 0.82 |
| > 2500 | 383 (30.2) | 2208 (53.0) | |||
| Education | < Voluntary education | 588 (46.6) | 1261 (30.3) | 2.80 (2.32-3.53) | < 0.01 |
| > Voluntary education | 672 (53.3) | 2904 (69.7) | |||
| Residence | Urban area | 569 (44.9) | 2184 (52.4) | 1.95 (1.64-2.31) | < 0.01 |
| Rural area | 698 (55.1) | 1982 (47.6) | |||
| Labor | Non-manual labor | 804 (63.5) | 3397 (81.5) | 6.9 (5.66-8.47) | < 0.01 |
| Manual labor | 463 (36.5) | 769 (18.5) | |||
| Physical-exercise frequency | < 8 times per month | 883 (69.7) | 3120 (74.9) | 0.56 (0.47-0.68) | < 0.01 |
| ≥ 8 times per month | 384 (30.3) | 1046 (25.1) | |||
| Hyperlipemia | No | 930 (73.4) | 3860 (92.7) | 1.99 (1.56-2.53) | < 0.01 |
| Yes | 337 (26.6) | 306 (7.3) | |||
| Heart disease | No | 1208 (95.3) | 4145 (99.7) | 1.03 (0.56-1.89) | 0.93 |
| Yes | 59 (4.7) | 21 (0.3) | |||
| Nervous system disease | No | 1224 (96.6) | 4114 (98.8) | 0.31 (0.18-0.55) | < 0.01 |
| Yes | 43 (3.4) | 52 (1.2) | |||
| diabetes | No | 1114 (87.9) | 4060 (97.5) | 1.69 (1.20-2.38) | 0.003 |
| Yes | 153 (12.1) | 106 (2.5) | |||
| Times of Nocturia | < 2 | 601 (47.5) | 3674 (88.6) | 8.2 (6.72-10.01) | < 0.01 |
| > 2 | 665 (52.5) | 472 (11.4) | |||
| Constipation | No | 740 (58.4) | 3118 (74.8) | 7.53 (6.01-9.43) | 0.002 |
| Yes | 527 (41.6) | 1048 (25.2) | |||
| Alcohol drinking | < 2 times/week | 1228 (96.9) | 4081 (98.0) | 1.33 (0.72-2.47) | 0.366 |
| > 2 times/week | 39 (3.1) | 85 (2.00) | |||
| Smoking | < 40 times/week | 1247 (98.4) | 4131 (99.2) | 1.80 (0.73-4.45) | 0.206 |
| > 40 times/week | 20 (1.6) | 35 (0.8) | |||
| Greasy food eating | Everyday | 176 (13.9) | 748 (18) | 0.80 (0.73-0.88) | < 0.01 |
| > 3 times/week | 417 (32.9) | 1471 (35.3) | |||
| > 1 times/week | 363 (28.7) | 1176 (28.2) | |||
| < 1 times/week | 311 (24.5) | 771 (18.5) | |||
| Respiratory disease | No | 1081 (85.3) | 3914 (94.0) | 2.67 (2.19-3.27) | < 0.01 |
| Yes | 186 (14.7) | 252 (6.0) | |||
| Marital status | Divorced or Widowed | 197 (15.5) | 259 (6.2) | 2.07 (1.59-2.70) | < 0.01 |
| Unmarried | 3 (0.2) | 556 (13.3) | 0.14 (0.44-0.45) | 0.01 | |
| Married | 1067 (84.2) | 3351 (80.4) | |||
Gynecological risk factors were analyzed independently using multivariate analysis (Table 5). The results indicate that menstruation is a protective factor for UI (OR = 0.18, 95% CI 0.15-0.20, P < 0.01). Women with frequent urinary tract infection (OR = 7.75, 95% CI 5.06-11.89, P < 0.011), pelvic organ prolapse (OR = 7.37, 95% CI 3.68-14.76, P < 0.011), and chronic pelvic pain (OR = 2.94, 95% CI 2.20-3.91, P < 0.01) have an increased risk of developing UI.
Table 5.
Multivariate Analysis of Gynecological Risk Factors of Participants
| Factories | UI case (%) | Control (%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Age of menopause | No | 448 (35.4) | 3103 (74.5) | 0.18 (0.15-0.20) | < 0.01 |
| < 45 | 102 (8.1) | 134 (3.2) | 0.99 (0.75-1.31) | 0.936 | |
| > 45 | 717 (56.6) | 929 (22.3) | Reference | ||
| Pelvic organ prolapse | No | 1234 (97.4) | 4153 (99.7) | 7.37 (3.68-14.76) | < 0.01 |
| Yes | 33 (1.3) | 13 (0.3) | Reference | ||
| Chronic pelvic pain | No | 1142 (90.1) | 4033 (96.8) | 2.94 (2.20-3.91) | < 0.01 |
| Yes | 125 (9.9) | 133 (3.2) | Reference | ||
| Urinary tract infection frequency | No | 1196 (94.4) | 4128 (99.0) | 7.75 (5.06-11.89) | < 0.01 |
| Yes | 71 (5.6) | 40 (1.0) | Reference | ||
There are associations between the mode of delivery and the prevalence rate of UI, according to our results. Univariate analysis indicates that cesarean section (OR = 0.42, 95% CI 0.36-0.49, P < 0.01) and vaginal delivery with episiotomy (OR = 0.19, 95% CI 0.15-0.239, P < 0.011) are protective factors for UI compared with vaginal delivery without episiotomy. Prolonged labor (OR = 7.78, 95% CI 5.11-11.86, P < 0.01) and pregnancy (OR = 2.50, 95% CI 2.10-2.97, P < 0.01), especially multiple pregnancies (OR = 8.31, 95% CI 6.86-10.06, P < 0.01), all increase the risk of UI.
Discussion
This study evaluated the prevalence of UI in Chinese women in Shanghai using a multi-step, community-based survey. A total of 5,467 Shanghai women were selected to participate in the cross-sectional survey. To our knowledge, this is the first population-based study addressing the issue of urinary incontinence in a metropolis as economically developed as Shanghai.
The overall prevalence of urinary incontinence in women in Shanghai is 23.3%. This is consistent with other published studies on this subject conducted in various areas in East Asia, the results of which range between 20 and 42% [6-9]. The prevalence rate of urinary incontinence in Shanghai is higher than that in Beijing (22.1%) [10]. Such a finding is not unexpected because over 16% of Shanghai residents are age 65 years and older [11]. The prevalence rates of SUI, UUI, and MUI are 12.9% (394/3058), 1.7% (52/3058), and 7.5% (229/3058), respectively. These data are similar to the results of the meta-analysis study by Hampel et al [12]. In addition, 21 out of 48 studies have indicated that nearly half of the women with UI are suffering from the symptoms of SUI.
Our study focuses on the risk factors of UI in Chinese women. It shows that aging can increase the risk of developing UI, which has also been noted in previous studies [13-15]. In our study, the prevalence rates of SUI, UUI, and MUI increase with age and peak between the age of 80 and 100 years old. The correlation of prevalence rate and age display two notable periods: the prevalence rate of SUI has a sharp increase in participants aged 40 to 49 years, whereas the prevalence rate of MUI displays a sharp increase in the participants aged 70 to 79 years. Hunskaar et al [16] reviewed several studies and found that the prevalence of UI by age displays an intriguing pattern of an early prevalence peak in midlife and then a steady increase among the elderly population. Hannestad et al [17] found a gradual increase of prevalence across adulthood until the age of 50 followed by a steady period, or even a slight decrease until age of 70, when prevalence started to increase again. The impact of aging on UI is attributed to many factors, including physiological and structural changes of the urinary tract such as abnormalities of detrusor contractility, problems with bladder elasticity, and mechanical and functional problems of the sphincter [18].
In addition, age-related factors, in addition to issues of the urinary tract, such as diabetes, hyperlipemia, constipation, and respiratory disease play a notably important role, as well. Diabetes is an important risk factor in our study, which is similar to a study by Hsieh et al [19]. Diabetes increases the risk of lower urinary tract infection and tends to cause an overactive bladder by vascular neuropathy [20]. Hyperlipemia has been found to be associated with a high prevalence rate of UI in many studies. However, the cause is uncertain. For example, maximal urethral closure pressure and blood supply of urethra is not correlated with hyperlipemia, as reported by Hall et al [21]. Subjects in our study with urinary incontinence displayed a high incidence of respiratory disease such as chronic cough, which is consistent with findings from other studies. Constipation and respiratory disease are highly associated with urinary incontinence because of high abdominal pressure, which increases the pressure of urethral and influences pelvic support tissue [22].
Univariate analysis in this study indicates that high BMI (≥ 28 kg/m2) can increase the risk of developing UI, which has also been reported in some previous studies. Nevertheless, BMI as a risk factor was rejected in the multivariate analysis in our study. It is still uncertain if high BMI can lead to the development of UI. Bump reported that high BMI caused a high abdominal pressure, which burdened pelvic floor muscle [23]. Customized pelvic support muscle exercise has been suggested to decrease the rate of SUI [24]. Women with high BMI tend to perform little physical exercise, which would increase the risk of UI. In fact, many risk factors and comorbid conditions associated with UI can be offset by a healthy life style, frequent physical exercise, and weight control.
This study strongly suggests that factors such as monthly income, educational background, heavy labor, and residential environment have a great impact on developing UI, which has also been reported in other studies. Heavy labor is identified as a possible risk factor for UI as it burdens pelvic floor muscles [25]. A poor living environment and low educational background may force residents to choose occupations that involve intense manual labor. It is also not a coincidence that this population tends to report a lower monthly income. In addition, in some rural districts of Shanghai, tub commodes are not widely used, which might contribute to a higher prevalence of UI in these areas. Clinical studies should be performed to confirm that tub commodes could reduce the prevalence rate of urinary incontinence. Conversely, people with a high living standard are inclined to care about their personal health. Such people are more likely to take preventive measures to maintain their well-being, which will have positive effect on reducing the risk for UI.
In our study, cesarean section and vaginal delivery with episiotomy were identified as protective factors for UI. These findings may be because women either receiving cesarean section or undergoing vaginal delivery with episiotomy suffer minor injury in pelvic support tissue after delivery. However, women who underwent vaginal delivery without episiotomy may be more likely to have laceration in pelvic support tissue of birth canal. Pregnancy history was identified as a risk factor for UI, which has also been reported in other studies. Pregnancy can lead to anatomic and physiologic changes of pelvic support tissue, which is the biological basis for preventing the occurrence of UI [26]. In this study, we observed that multiple pregnancies are a significant risk factor developing UI, which may be a result of the repeated injuries in pelvic support tissue.
We found the menopause was significantly correlated with the development of UI. This correlation may be observed because menopause affects estrogen production. Normal physiological functions of the lower urinary tract could be impaired by the shortage of estrogen [27].
Gynecological disease, chronic pelvic pain and urinary infection were associated with UI in our study. Lower urinary tract is innervated by pelvic parasympathetic nerves, lumbar sympathetic nerves, and pudendal nerves. Abnormal stimulation triggered by the above disorders of these nerves may lead to the dysfunction of the lower urinary tract [28].
Ten questions were created in our questionnaire to assess the quality of sexual activities of the participants, but general reluctance to answer these questions prevented us from further analyzing this aspect in our study. Because of Chinese social and cultural norms, a large number of women with UI might be embarrassed to consult physicians with such conditions. As a result, the true prevalence rate of UI in Shanghai women could be possibly considerably higher.
This study has several limitations. First of all, our results regarding the urinary incontinence diagnosis and its subtypes are based only on the participants’ answers in the questionnaires. Therefore, it is subject to information bias, which might influence its accuracy to a certain extent. In addition, further studies should be conducted to evaluate the relationship between the quality of life and urinary incontinence of Shanghai women. Nevertheless, we believe that this study has provided a unique opportunity to evaluate urinary incontinence and may facilitate possible interventions in the future.
Conclusion
UI is a common disorder in Shanghai Chinese women, and many risk factors may affect the development of UI. Aging, overweight, lack of education, late menarche, menstrual disorder, pregnancy history, episiotomy, CPP, POP, gynecological disease, other chronic diseases, constipation, fecal incontinence, urinary tract infection, and nocturia are a few examples of such factors. We learned that most Chinese women did not have any knowledge of UI, and they lived with the disease without early diagnosis and proper treatments.
Acknowledgements
We are grateful to each resident for the cooperation, and also we thank Dr Zi-Shen Ai from Tongji University for providing expert technical assistance on SPSS. We are grateful to Dr Deng-Long Wu for his help with the funding in this study.
Disclosure of conflict of interest
None.
References
- 1.Abrams P, Avery K, Gardener N, Donovan J. The International Consultation on Incontinence Modular Questionnaire: http://www.iciq.net. J Urol. 2006;175:1063–1066. doi: 10.1016/S0022-5347(05)00348-4. discussion1066. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93:324–330. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 3.Perry S, Shaw C, Assassa P, Dallosso H, Williams K, Brittain KR, Mensah F, Smith N, Clarke M, Jagger C, Mayne C, Castleden CM, Jones J, McGrother C. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the LeicestershireMRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med. 2000;22:427–434. doi: 10.1093/pubmed/22.3.427. [DOI] [PubMed] [Google Scholar]
- 4.Vandoninck V, Bemelmans BL, Mazzetta C, Robertson C, Keech M, Boyle P, Kiemeney LA. The prevalence of urinary incontinence in community-dwelling married women: a matter of definition. BJU Int. 2004;94:1291–1295. doi: 10.1111/j.1464-410X.2004.05214.x. [DOI] [PubMed] [Google Scholar]
- 5.Hilde G, Staer-Jensen J, Siafarikas F, Ellstrom Engh M, Bo K. Postpartum pelvic floor muscle training and urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2013;122:1231–1238. doi: 10.1097/AOG.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt N, Ozkan S, Korucuoglu U, Onan A, Aksakal N, Ilhan M, Himmetoglu O. Urogenital symptoms of postmenopausal women in Turkey. Menopause. 2007;14:150–156. doi: 10.1097/01.gme.0000227857.12356.1e. [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Sung HH, Na S, Choo MS. Prevalenceof urinary incontinence in Korean women:results of a National Health Interview Survey. World J Urol. 2008;26:179–185. doi: 10.1007/s00345-008-0239-2. [DOI] [PubMed] [Google Scholar]
- 8.Manonai J, Poowapirom A, Kittipiboon S, Patrachai S, Udomsubpayakul U, Chittacharoen A. Female urinary incontinence: a cross-sectionalstudy from a Thai rural area. Int UrogynecolJ Pelvic Floor Dysfunct. 2006;17:321–325. doi: 10.1007/s00192-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 9.Tsai YC, Liu CH. Urinary incontinence among Taiwanese women: an outpatient study of prevalence, comorbidity, risk factors, and quality of life. Int Urol Nephrol. 2009;41:795–803. doi: 10.1007/s11255-009-9523-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Lang J, Liu C, Han S, Huang J, Li X. The epidemiological study of women with urinaryincontinence and risk factors for stress urinary incontinence in China. Menopause. 2009;16:831–836. doi: 10.1097/gme.0b013e3181967b5d. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Zhu G, Zhao Q, Guo Q, Meng H, Hong Z, Ding D. Prevalence and Risk Factors of Poor Sleep Quality among Chinese Elderly in an Urban Community: Results from the ShanghaiAging Study. PLoS One. 2013;8:e81261. doi: 10.1371/journal.pone.0081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampel C, Wienhold D, Benken N, Eggersmann C, Thuroff JW. Definition of overactivebladder and epidemiology of urinary incontinence. Urology. 1997;50:4–14. doi: 10.1016/s0090-4295(97)00578-5. discussion 15-17. [DOI] [PubMed] [Google Scholar]
- 13.Botlero R, Davis SR, Urquhart DM, Shortreed S, Bell RJ. Age-specific prevalence of, and factors associated with, different types of urinaryincontinence in community-dwelling Australianwomen assessed with a validated questionnaire. Maturitas. 2009;62:134–139. doi: 10.1016/j.maturitas.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Onur R, Deveci SE, Rahman S, Sevindik F, Acik Y. Prevalence and risk factors of female urinary incontinence in eastern Turkey. Int J Urol. 2009;16:566–569. doi: 10.1111/j.1442-2042.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 15.Azuma R, Murakami K, Iwamoto M, Tanaka M, Saita N, Abe Y. Prevalence and risk factors of urinary incontinence and its influence on the quality of life of Japanese women. Nurs Health Sci. 2008;10:151–158. doi: 10.1111/j.1442-2018.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- 16.Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62:16–23. doi: 10.1016/s0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 17.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 18.Klauser A, Frauscher F, Strasser H, Helweg G, Kolle D, Strohmeyer D, Stenzl A, zur Nedden D. Age-related rhabdosphincter function in female urinary stress incontinence: assessment of intraurethral sonography. J Ultrasound Med. 2004;23:631–637. doi: 10.7863/jum.2004.23.5.631. quiz 638-639. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CH, Lee MS, Lee MC, Kuo TC, Hsu CS, Chang ST. Risk factors for urinary incontinence in Taiwanese women aged 20-59 years. Taiwan J Obstet Gynecol. 2008;47:197–202. doi: 10.1016/S1028-4559(08)60080-7. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733–738. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 21.Hall R, Kkhalsa S, Qualls C, Rogers RG. A comparison of periurethral blood flow resistive indices and urethral closure pressure of incontinent women. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:472–477. doi: 10.1007/s00192-005-0044-z. [DOI] [PubMed] [Google Scholar]
- 22.Al-Badr A, Brasha H, Al-Raddadi R, Noorwali F, Ross S. Prevalence of urinary incontinence among Saudi women. Int J Gynaecol Obstet. 2012;117:160–163. doi: 10.1016/j.ijgo.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Bump RC, Sugerman HJ, Fantl JA, McClish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167:392–397. doi: 10.1016/s0002-9378(11)91418-5. discussion 397-399. [DOI] [PubMed] [Google Scholar]
- 24.Nygaard IE. Does prolonged high-impact activity contribute to later urinary incontinence? A retrospective cohort study of female Olympians. Obstet Gynecol. 1997;90:718–722. doi: 10.1016/S0029-7844(97)00436-5. [DOI] [PubMed] [Google Scholar]
- 25.Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22:127–135. doi: 10.1007/s00192-010-1215-0. [DOI] [PubMed] [Google Scholar]
- 26.O’Boyle AL, O’Boyle JD, Ricks RE, Patience TH, Calhoun B, Davis G. The natural history of pelvic organ support in pregnancy. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:46–49. doi: 10.1007/s00192-002-1006-3. discussion 49. [DOI] [PubMed] [Google Scholar]
- 27.Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. 2003;62:45–51. doi: 10.1016/s0090-4295(03)00676-9. [DOI] [PubMed] [Google Scholar]
- 28.Silva WA, Karram MM. Anatomy and physiology of the pelvic floor. Minerva Ginecol. 2004;56:283–302. [PubMed] [Google Scholar]


