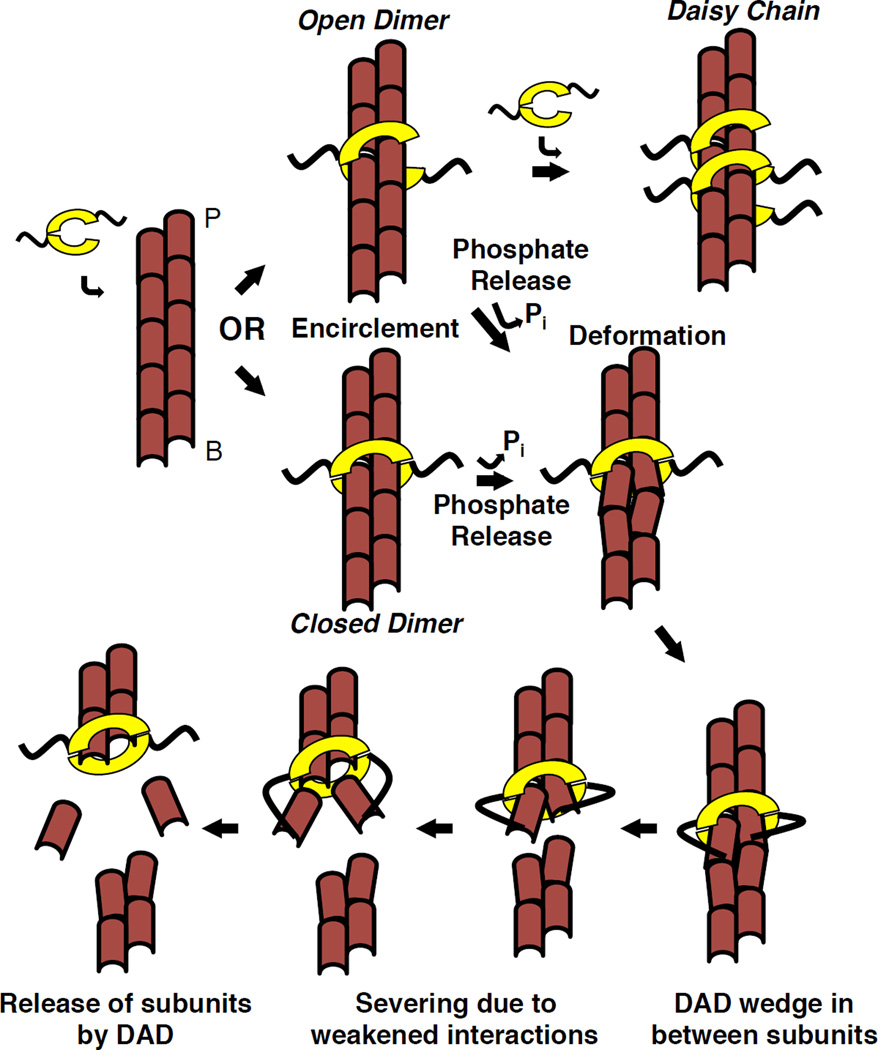
Figure 6. Model of INF2 severing mechanism.
INF2 dimer partially dissociates, then encircles the filament, either as a closed ring or an open dimer that can form a daisy-chain. Subsequent steps shown in the closed ring conformation, but could also occur as the open dimer. INF2 binding causes a change in helical rise. Upon phosphate release, INF2 causes further local filament deformation, partially exposing the DAD binding site between subdomains 1 and 3 of adjacent actin protomers. DAD binding destabilizes the filament by wedging between protomers. These destabilizations lead to severing at this weak point. Upon severing, INF2 remains bound to the new barbed end, and DAD releases bound actin monomers removed from filament. Our data suggest that DAD binding to the barbed end side of the FH2 is favored.