Abstract
Background:
Cytokine-induced killer (CIK) cells are ex vivo-expanded immune cells that express NK-cell and T-cell markers and that are routinely used in the treatment of many cancers. One key advantage of CIK cells is their ability to efficiently traffic to many solid tumours. Although likely to be mediated by chemokine receptor (CKR) expression, a thorough examination of the mechanism of tumour targeting has not been previously explored.
Methods:
Here, human CIK cell expansions were examined for the level, profile and kinetics of CKR expression.
Results:
It was found that CIK cells express a panel of CKRs, with considerable variation between donors. Importantly, CKR levels dropped considerably beyond 14 days in culture, being significantly reduced by day 28 (the time at which cytolytic activity peaked). As such, CIK preparations that are used clinically may not have optimal CKR expression. Several approaches were found to re-stimulate CKR cell-surface levels at these later time points. These approaches also enhanced cytolytic activity in vitro and were demonstrated to increase both in vivo tumour trafficking and anti-tumour activity in mouse models.
Conclusions:
Simple modifications of the CIK expansion protocol could therefore be used to significantly enhance the anti-tumour effects of this therapy.
Keywords: chemokine, chemokine receptor, Cytokine-induced Killer (CIK) cell
An expansion protocol for the production of cytokine-induced Killer (CIK) cells was initially reported 20 years ago (Schmidt-Wolf et al, 1993; Lu and Negrin, 1994). However, despite widespread use in Asia and some promising clinical data targeting haematopoietic malignancies in the United States (Leemhuis et al, 2005), the treatment of solid tumours with this immune cell therapy has, to date, been disappointing (Belin et al, 2013; Zhao et al, 2013). This is primarily because of the limited cytolytic potential of this population. However, one of the greatest strengths of CIK-based therapies over similar cell types, such as LAK cells, is their capacity to traffic systemically to solid tumours, including residual disease (Sasaki et al, 1991; Scheffold et al, 2002). This tumour-targeting potential has been used in several recent pre-clinical reports where CIK cells have been used in combination with other immunotherapies or biological therapies to act as delivery vehicles or targeting agents, and this may lead to significant improvement in clinical responses (Thorne et al, 2006; Liu et al, 2013).
However, the basis for the tumour-directed trafficking capacity of CIK cells is poorly understood at present. It is likely that one important factor directing CIK cell trafficking would be the interaction of chemokines released from the tumour microenvironment with chemokine receptors (CKRs) on the CIK cell surface (Baker et al, 2001). However, a detailed analysis of CKR profiles on CIK cells has not been reported previously, and the effects of the expansion protocol on CKR expression are not known. It is predicted that a better understanding of the mechanisms used by CIK cells to traffic to a broad range of tumour types could ultimately be applied to other adoptive immune cell therapies.
It was discovered that, although a variety of CKRs are expressed on the surface of CIK cells and considerable donor-to-donor variability existed, one consistent factor was that CKR levels dropped during the later stages of the ex vivo expansion protocol. As a result, the levels of many CKRs were significantly reduced by the time the cytolytic activity peaked (the time at which CIK cells were typically reinfused back to patients in clinical settings). This observation might at least partially explain why CIK cell therapies have been less effective in clinic than predicted from pre-clinical models.
Approaches to boost cell-surface CKR levels at these later times were examined, and the effects of these on cytolytic activity and on in vivo tumour trafficking, as well as anti-tumour effects in mouse models, were determined.
Materials and methods
Cell lines
Human ovarian cancer cell line UCI-101-luc was a kind gift from Dr P DiSaia (Fuchtner et al, 1993). HELA-luc cell line was purchased from ATCC (Manassas, VA, USA) and transfected with a retrovioral plasmid expressing luciferase. Murine colon adenocarcinoma cell line MC38 was purchased from ATCC, and the MC38-luc cell line was derived from MC38 through transfection with a luciferase expression plasmid.
Human and mouse CIK cell culture
For human CIK cell culture, peripheral blood lymphocytes were obtained in accordance with guidelines set forth by the Institutional Review Board as buffy coats from the Pittsburgh Blood Bank (Pittsburgh, PA, USA). Briefly, lymphocytes were isolated from buffy coats by Ficol density gradient centrifugation. Mononuclear cells were cultured in 10% fetal calf serum/RPMI-1640 with interferon-γ (IFN-γ, ActImmune) added at the initiation of culture. OKT3 (Ortho Biotech, Raritan, NJ, USA) and interleukin-2 (IL-2; Proleukin) were added 24 h later, and the culture was maintained by the addition of fresh medium with IL-2 every 3 days. The CIK cells were harvested and assays were performed at the indicated times.
For mouse CIK cell culture, activated CIK cells were expanded from spleens of C57BL/6 mice or C57BL/6 GFP+ mice or C57BL/6 CXCR3−/− crossed with C57BL/6 GFP+. Briefly, splenocytes were cultured with IFN-γ and anti-CD3e antibody for 24 h. After 24 h, IL-2 was added. In the following days, the medium was changed as needed and fresh 1640 medium with IL-2 was added.
In some cases, additional treatments were applied to the CIK cells 24 h before their use. This included IL-12, anti-CD3/anti-CD25-coated beads and high doses of IL-2.
Recombinant human IL-12 was purchased from R&D Systems (Minneapolis, MN, USA), and the working concentration is 0.01 ng ml−1.
Human T-Activator CD3/CD28 and mouse T-Activator CD3/CD28 beads were purchased from Life Technologies (Grand Island, NY, USA). They were used according to the manufacturer's instructions and removed by magnet before using the CIK cells.
Flow cytometry
For human CIK cells, effector (CIK) cells harvested at different time points were washed, stained with various monoclonal antibodies for 2–4 h and analysed by flow cytometry. The following antibodies were used: PE mouse IgG1, k isotype control, PE mouse IgG2a, k isotype control, anti-CD3 FITC, anti-CD56 APC, anti-CCR4 PE, anti-CCR5 PE, anti-CXCR3 PE, anti-CXCR4 PE and anti-CCR7 PE. All the antibodies except anti-CCR7 were purchased from BD Pharmigen (Franklin Lakes, NJ, USA). The CCR7 antibody was purchased from R&D systems. All the antibodies were used according to the manufacturer's directions. Samples were analysed using a CYAN Flow Cytometer (Dako, Carpinteria, CA, USA), and analysis was with FlowJo (Treestar Inc., Ashland, OR, USA). In all cases, DAPI staining was used to determine viability and to gate for live cells.
For mouse CIK cells, the following antibodies were used: mouse anti-CD3 FITC, mouse anti-CD3 PE, mouse anti-CD3 APC, mouse anti-CCR5 FITC and mouse anti-CXCR3 PE. All of the antibodies were purchased from e-Bioscience company (San Diego, CA, USA). All the antibodies were used according to the manufacturer's directions. Samples were analysed using a CYAN Flow Cytometer (Dako) and analysed with FlowJo.
Cell killing assay
Human and mouse CIK cell cytotoxicity was measured using a luciferase expression-based CTL assay. For human CIK cells, effector (CIK) cells were harvested at 14 days, 21 days and 28 days. UCI-101-luc or Hela-luc cells were used as the target cells. For mouse CIK cells, effector cells were spleen CIK cell culture harvested at 7 or 14 days. MC38-luc was used as the target cell line. Effector cells were added to target cells at E : T ratios of 5 : 1, 10 : 1, 20 : 1 and 40 : 1 and left at 37 °C, 5% CO2 for 4 h. Every condition was repeated in triplicate. After 4 h, luciferin was added using 2 μl per well of a luciferin stock (30 mg ml−1) and an IVIS200 (Xenogen, caliper life sciences, Hopkintonm, MA, USA) was used to image and quantify the signal from the plate over ∼2 min. The percentage of surviving cells was quantified relative to untreated target cells (100% survival) or CIK cells only (0% survival).
Transwell assay
Mouse Recombinant IP-10 (BioLegend) was added into the lower wells of 3.0-μm-pore 96-well Transwell (Corning, Corning, NY, USA) plates, whereas 500 000 CIK cells were added into each of the upper wells. These cells were incubated at 37 °C for 3 h, and the numbers of migrated immune cells were counted by flow cytometry.
Human CIK expansions (day 28) were untreated or treated with CD3/CD28 beads for 24 h before addition to the top well of transwell plates. The indicated chemokines were applied in the lower wells, and migration (number of cells in lower plate) was determined by flow cytometry after 24 h.
Mouse models
Female albino C57BL/6 mice were purchased from Charles River (Wilmington, MA, USA). For biodistribution studies, C57BL/6 mice were injected s.c. with 200 000 MC38 cells. When the tumours reached 50–100 mm3, animals were regrouped and treatment was initiated. Mice were treated via intravenous tail-vein injection of 1 × 107 CIK cells or bead-treated CIK cells. CIK cells were labelled using cy5.5 NHS ester (Lumiprobe Corporation, Hallandale, FL, USA) half an hour before injection.
Cy5.5-labelled mCIK cells were imaged using the Fluorescence Molecular Tomography (FMT) 2500 system (Perkin Elmer) at 24, 48 and 72 h after injection of CIK cells. In some experiments, tumour volumes were measured as bioluminescence signal (for MC38-luc tumours; bioluminescence imaged on an IVIS200 (Xenogen)) or by using calipers. In some additional experiments, mice were killed and GFP signal was examined in tumour sections ex vivo using an Axiovert fluorescence microscope (Zeiss).
All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Statistical analysis
Paired t-test was used to determine statistical significance, defined as P<0.05.
Results
CIK cells express multiple chemokine receptors on their cell surface, and there is significant donor-to-donor variation
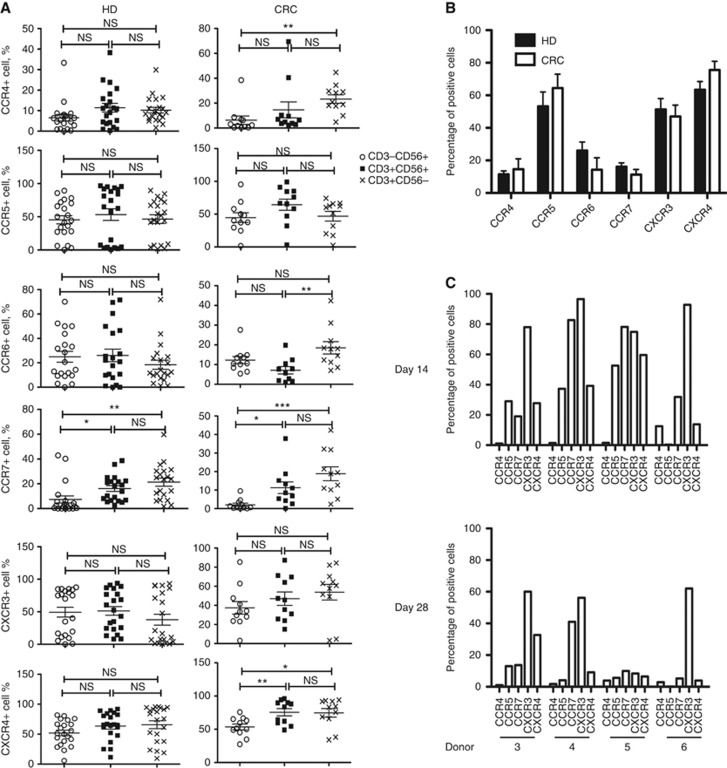
In initial studies, multiple CIK preparations were expanded from buffy coats obtained from healthy donors (n=28) and colorectal cancer (CRC) patients (n=11). Our previous studies and those of other groups (Baker et al, 2001; Marin et al, 2006) have determined that 6 chemokine receptors are expressed on CIK cells (CCR4, CCR5, CCR6, CCR7, CXCR3 and CXCR4). In addition, because CIK cells represent a mix of NK (CD3−CD56+), NK-T (CD3+CD56+) and T cells (CD3+CD56−), it was important to determine the relative abundance of CKR on the different cell lineages.
It was seen (Figure 1A) that, in general, chemokine receptor levels were higher in T-cell and NK-T-cell populations relative to NK cells (which is in agreement with previous studies showing that these populations are more effective at trafficking to the tumour target (Schmidt-Wolf et al, 1993; Nishimura et al, 2008)). In addition, CCR5, CXCR3 and CXCR4 were the most highly expressed chemokines in the CD3+CD56+ population (with 55, 52 and 62% of CD3+CD56+ cells positive for these CKRs, and 12, 27 and 17% positive for CCR4, CCR6 and CCR7, respectively). However, differences between the cell lineages and between healthy and CRC patient donors were rarely significant (Figure 1B), primarily because of the high degree of variability between individual donors. Several examples of individual CKR profiles (for CD3+CD56+ populations) are shown for four different healthy donors in Figure 1C, showing the variability in CKR profiles between donors. No correlation between age or sex and CKR profile was observed, and the number of CIK cell samples was not large enough to determine whether distinct patterns of CKR profiles were being reproduced.
Figure 1.
CKR profiles on human CIK cell expansions. (A) Healthy donor (HD; n=28) and colorectal cancer patient (CRC; n=11) CIK cells at day 14 were stained for NK (CD56) and T-cell (CD3) markers, as well as the 6 chemokine receptors known to be expressed on CIK cells (CCR4, CCR5, CCR6, CCR7, CXCR3, CXCR4). Percentages of cells of different cell types (CD3−CD56+ CD3+CD56+ CD3+CD56−) positive for different CKRs are shown. (B) Comparisons of CKRs on CD3+CD56+ CIK cells from HD and CRC (no significant differences seen for any CKR; N.B. Other differences between these groups include average age (HD=39 years; CRC=53 years) and sex (HD=29% female; CRC=45% female). (C) Differences in CKR profiles in CD3+CD56+ CIK cell populations between donors (for 4 donors) and at different times after expansion are shown. Individual donors show distinct patterns of CKR profiles, which are gradually reduced over time in culture.
Surprisingly, the majority of the CIK cell expansions expressed most or all of the six CKRs examined on the surface of at least some of the CD3+CD56+ cells. This panel of chemokine receptors covers a broad spectrum of chemokine targeting patterns, and it is possible that this is critical in allowing CIK cells to effectively traffic to multiple different solid tumour types, as has been observed in pre-clinical models (Scheffold et al, 2002).
However, the considerable variability in the relative percentages of cells positive for the different CKRs, among CIK cell preparations from different donors (Figure 1C), may be important in explaining the inconsistent responses seen in the treatment of solid tumours. Although there was also some variation in cytolytic activity of the different CIK cell expansions, this was considerably less pronounced than that seen for CKR expression levels.
CKR profiles remain relatively constant over the expansion process, but levels drop after 14 days in culture
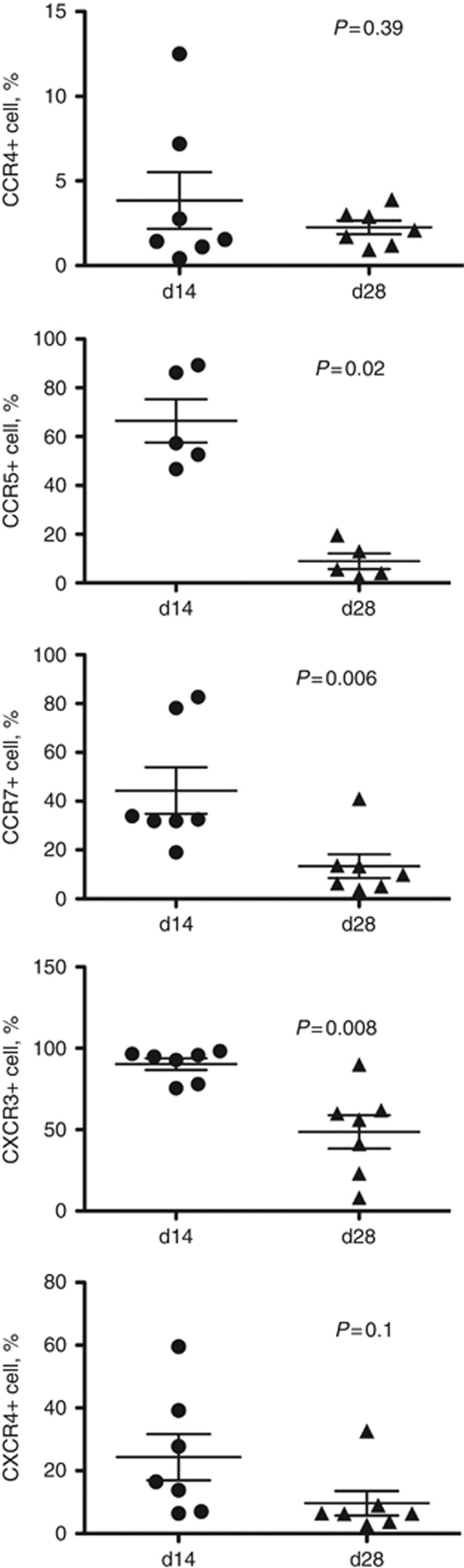
To determine whether this variation in CKR profile was due to donor variability, variability in the timing of sampling or issues related to the expansion process (such as timing since last split of the cells, cell density or numbers in a flask etc.), CIK cells from the same donor were profiled multiple times during the expansion process (this was examined for 7 of the healthy donors) (Figures 1C and 2). It was apparent that variability in CKR profiles primarily existed between donors, and that the relative expression of different CKRs for any individual donor remained relatively constant during the expansion process (Figure 1C).
Figure 2.
CKR expression is lost over time in culture. The levels of each CKR (CCR4, CCR5, CCR7, CXCR3 and CXCR4 shown as the percentage of cells positive in the preparation relative to isotype control) are shown at days 14 and 28 for each expansion. A trend towards reduced CKR expression is seen for all CKRs studied; this is significant (P<0.05, paired t-test) for CCR5 (P=0.0003), CCR7 (0.013) and CXCR3 (0.0024).
However, it was also observed that the level of cell-surface expression of the majority of the CKRs examined dropped during the course of CIK expansion, peaking at about day 14 in culture and demonstrating considerable subsequent reduction by day 28. This was again found to be reproducible for multiple donors (Figures 1C and 2). This apparent exhaustion of CKR expression may be important, as CIK reinfusions to patients are typically not performed until 28 days in culture. This observation may help explain why CIK cells used against solid tumours in the clinic have been less effective than expected based on pre-clinical results.
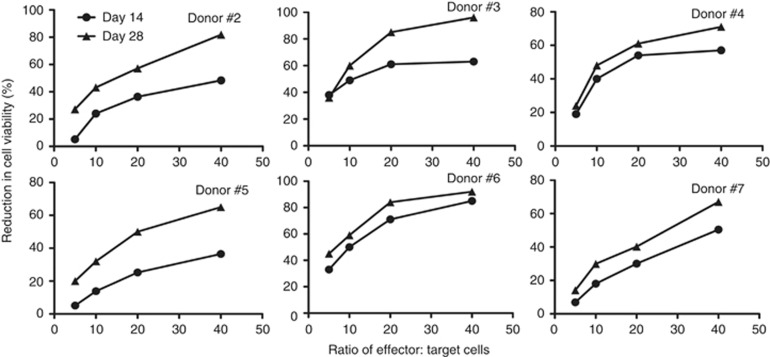
Cytolytic activity of CIK cells peaked at 28 days in culture
To determine whether the observed loss of CKR after 14 days in culture correlated with a loss of overall cytolytic activity of the CIK cells, the same preparations were used in basic CTL assays against several human tumour cell lines at different times during the expansion process (Figure 3). It was seen that although CKR cell-surface levels peaked at day 14, the cytotoxic capability of the cells did not reach maximum levels until day 28. This disconnect means that CIK cells used against solid tumours where trafficking of the cells to the tumour is crucial might be better used after a shorter expansion period, especially if the CIK cells are being primarily incorporated as a viral or gene delivery vehicle.
Figure 3.
Changes in CIK cell cytotoxicity between days 14 and 28 in culture. CTL assays against HeLa cells expressing luciferase (and UCI-101, data not shown) are shown for six CIK expansions. In all cases examined, the CTL assays demonstrate increased cytotoxicity at day 28 relative to day 14.
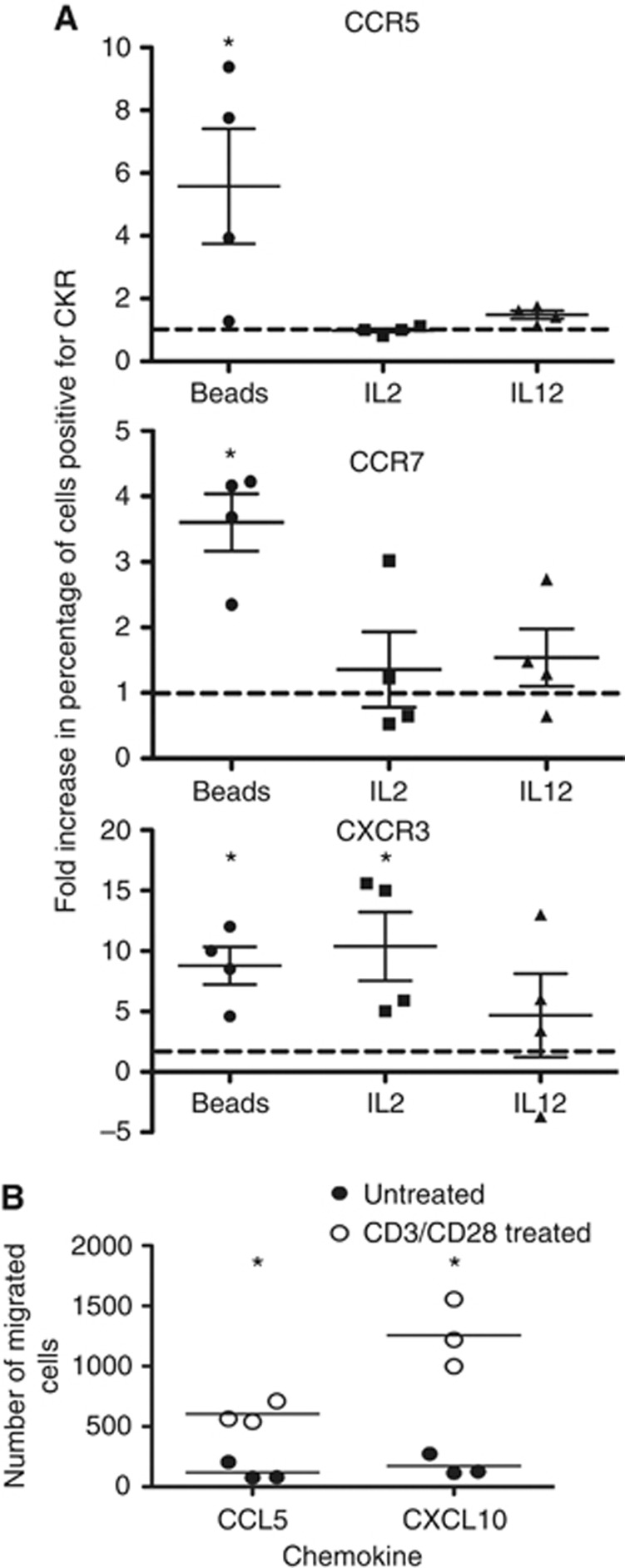
CKR cell-surface expression on CIK cells can be boosted through the addition of different immunostimulants
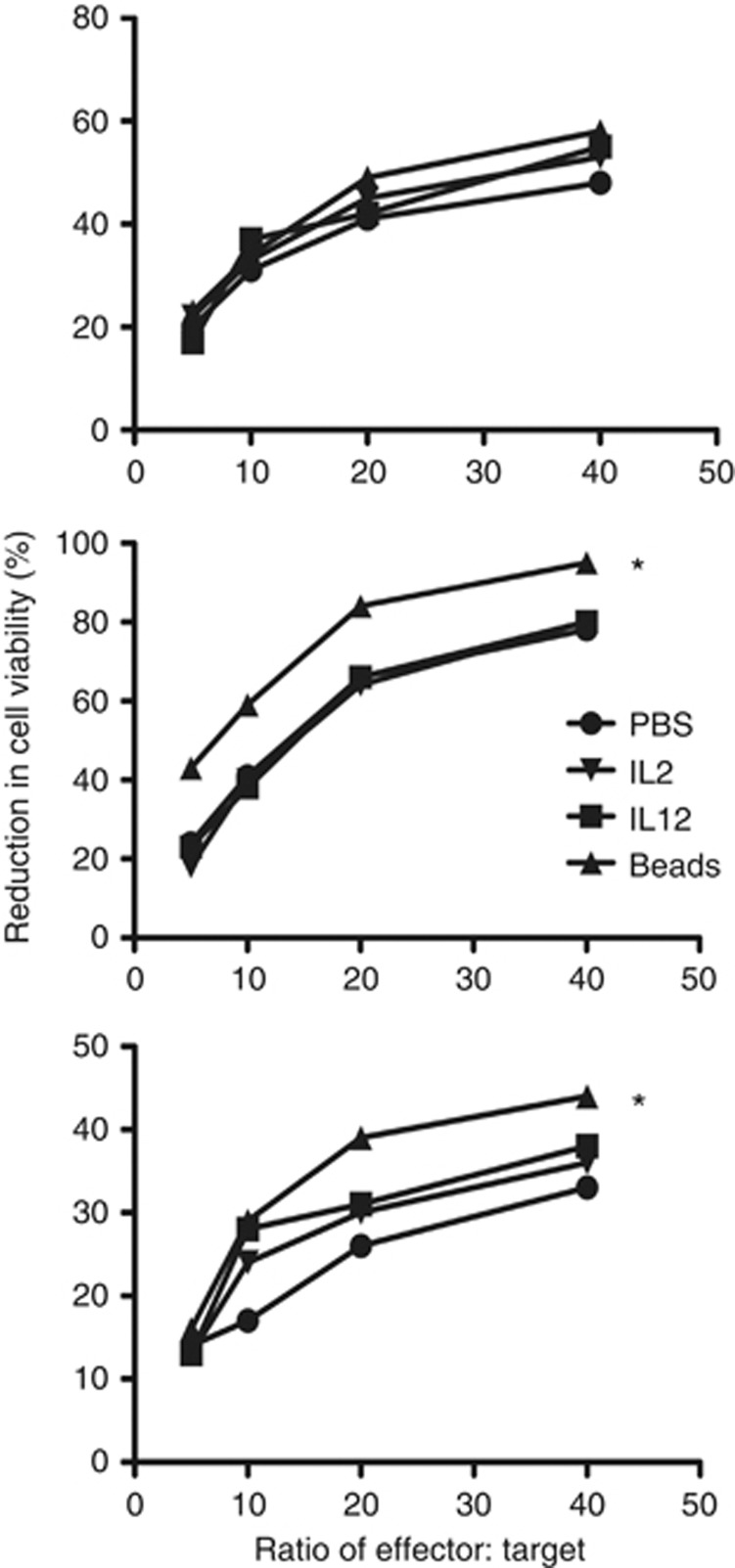
To determine whether it is possible to boost the CKR cell-surface expression levels at the later time points (28 day expansion), a series of immune stimulants were added to the CIK preparations at this time point. These treatments included adding IL-12 or a high dose of IL-2 for 24 h, or the addition of anti-CD3/anti-CD28-coated beads to the culture for 24 h. It was observed (Figure 4) that any of these treatments resulted in some level of re-stimulation of CKR expression in at least some CIK cell preparations (when the 3x CKR that showed significant decreases between day 14 and day 28 (CCR5, CCR7 and CXCR3) were examined in CD3+CD56+ populations). However, the use of anti-CD3/anti-CD28-coated beads provided the most consistent and significant results with regard to re-expression of these CKRs. This was further supported with cell migration assays (Figure 4), demonstrating that anti-CD3/anti-CD28 beads also markedly enhanced the trafficking of CIK cells (after 28 days in culture) towards appropriate chemokines, including CCL5 (that binds CCR5) and CXCL10 (that binds CXCR3).
Figure 4.
Effects of different immune stimulants at day 28 in culture on CKR levels. (A) Several of the expansions were treated with different immune stimulants (IL-12, high-dose IL-2 or exposure to CD3/CD28-coated beads for 24 h), and the effects on the overall levels (percentage of positive cells) of the three different CKR that displayed significant reductions between days 14 and 28 (CCR5, CCR7, CXCR3) were re-determined by flow cytometry, as described earlier. Fold increase in the percentages of positive cells relative to untreated controls at the same time are shown. In all cases, CKR levels were increased, with CD3/CD28-coated beads producing the most consistent and reliable increases in all three CKR levels. (B) Cell migration assay. In additional experiments, CIK expansions (day 28) were treated with CD3/CD28 beads for 24 h before addition to the top well of transwell plates. The indicated chemokines were applied in the lower wells, and migration (number of cells in lower plate) was determined by flow cytometry after 24 h (*P<0.05 for paired t-test comparing percentage positive with or without treatments).
The addition of anti-CD3/anti-CD28-coated beads during the final steps of the expansion protocol therefore provides a possible approach to enhancing the effectiveness of CIK cells in the clinic. However, it is important to ensure that this does not reduce the overall cytotoxic capabilities of the CIK cells. This was therefore examined (Figure 5). It was seen that the addition of anti-CD3/anti-CD28-coated beads to the CIK culture did not reduce cytotoxicity in any of the preparations tested and actually enhanced the killing in some cases. This modification of the expansion protocol therefore has the potential to provide an advantage in both trafficking and tumour killing.
Figure 5.
Cytotoxicity assay for CIK expansions (28 days) treated with different immune stimulants as before. No treatment had a negative effect on CIK cellular cytotoxicity, with CD3/CD28-coated beads producing an increase in CTL cytotoxicity for 2 of the 3 CIK expansions shown (*P<0.05 relative to PBS control).
Mouse CIK cell expansions also display CKR ‘exhaustion' during the expansion profile
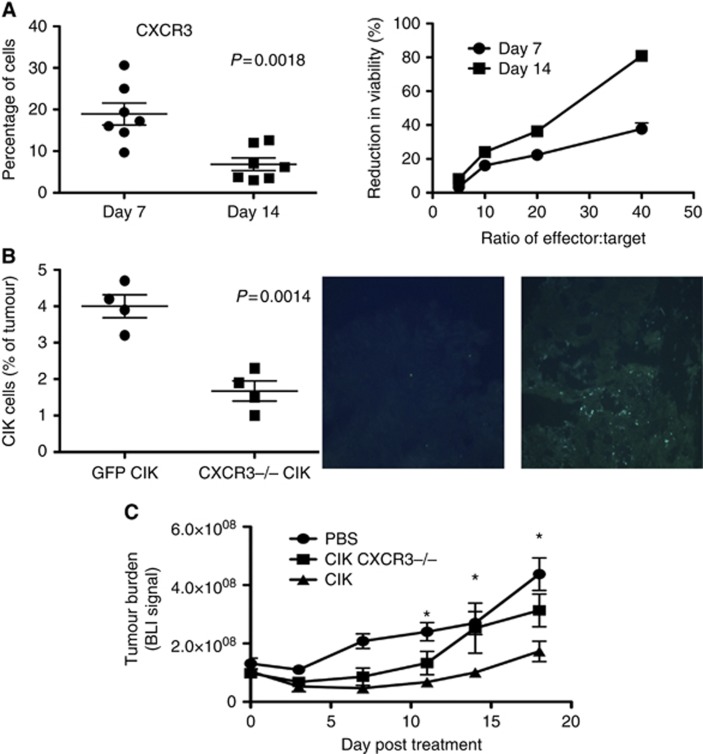
CIK cells expanded from mouse splenocytes have been used as a model of CIK cell therapies for pre-clinical research (Baker et al, 2001); however, mouse CIK cells demonstrate a shorter expansion process (with cells becoming quiescent by day 28, and thus they are typically reinfused at day 14). It was therefore examined whether mouse CIK cells display similar reductions in CKR levels as seen with hCIK cells. Indeed, in a situation analogous to human CIK cells, murine CXCR3 expression levels also dropped over the course of the expansion process, with CKR expression peaking at earlier times (Figure 6A). However, there were several key differences in the mouse models; (i) in general, the mouse CIK cells expressed fewer CKRs on their surface, with CXCR3 being the only CKR that could be reliably and reproducibly detected on the cell surface by flow cytometry (N.B. Our qRT-PCR results indicated that other CKRs are being expressed in mCIK cells, including CCR5, but it was unclear whether these were not expressed on the surface or whether reliable antibodies were not available); (ii) the peak of CKR expression occurred at around day 7, whereas CTL activity peaked at day 14 (by which point CXCR3 expression levels were significantly reduced) (Figure 6A), meaning that the same events occurred as for human CIK cells, but over a shorter time frame in the mouse model; (iii) the expansion of different CIK cell preparations from the same mouse strain produced consistent CKR profiles, but there was some variation if different strains were used, again implying that variation in CKR profiles is donor dependent.
Figure 6.
CKR profiles and kinetics in mouse models. (A) Mouse CIK cells also displayed a reduction in CKR levels, only over a shorter time in culture (day 10 to day 14), and for a smaller range of CKR (CXCR3 appears critical); data shown are for percentages of CXCR3-positive cells in mCIK cultures expanded from C57/BL6 mice at days 7 and 14 (n=7, reduction is significant, P=0.0018). CTL assay-determined cellular cytotoxicity also increased over the same time period (MC38-luc cells used as target) (data is average from n=3 preparations). (B) Loss of CXCR3 reduces trafficking of CIK cells to their tumour targets and reduced anti-tumour effects in vivo. Mice (C57/BL6) bearing subcutaneous tumours (MC38-luc of 50–100 mm3) were treated with intravenous (tail vein) injection of CIK cells (1 × 107 cells) expanded from a C57BL6 CXCR3−/− and GFP+ transgenic mouse or from a GFP+ strain (n=4 per group). Subsequent tumour trafficking was determined both by flow cytometry on dissociated tumours (left) and through examination of GFP staining in tumour sections post mortem. Significantly less CXCR3−/− CIK cells were found in the tumours, P=0.0014); (C) Anti-tumour effects as determined by bioluminescence imaging of MC38-luc cells were determined in the same model (n=6 per group). *CIK cells produced significantly enhanced therapeutic effects relative to all other groups on days 11, 14 and 18 (P<0.05).
CXCR3 is critical for mCIK trafficking to tumours
To examine how critical CKR expression is to CIK activity, CIK cells were expanded from a CXCR3−/− mouse and infused back into wild-type (C57/BL6) mice bearing s.c. colorectal tumours (MC38). The CXCR3−/− CIK cells were also labelled with GFP for the easy identification and quantification of adoptively transferred cells. It was seen (Figure 6B) that CXCR3 was important for mCIK tumour trafficking in this model, and that loss of CXCR3 resulted in a significant reduction in tumour infiltration by the CIK cells and a loss of anti-tumour activity (Figure 6C). This confirms that CKR expression is needed for trafficking and thus for in vivo therapeutic activity of CIK cells.
Boosting CKR levels in mouse models enhances cell trafficking
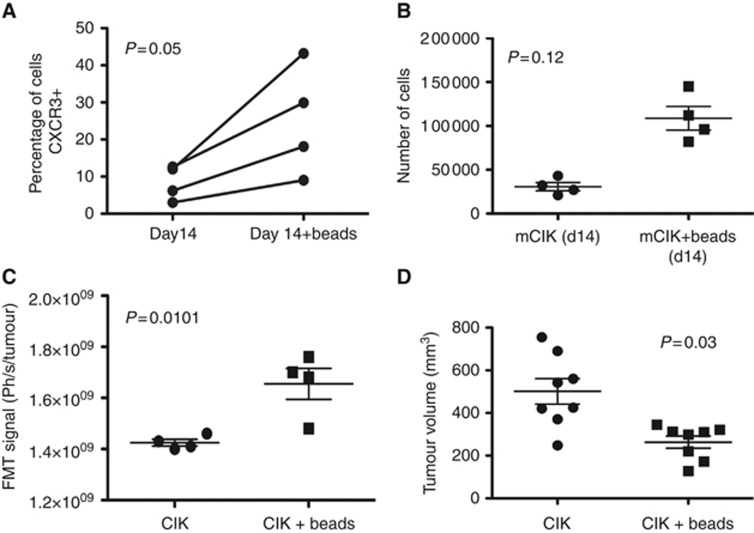
To determine whether the addition of immune-stimulating factors such as anti-CD3/anti-CD28-coated beads can also boost CKR levels in mouse models, and to examine how this influenced in vivo activity, mouse CIK cells at 14 days of expansion were treated with anti-CD3/anti-CD28-coated beads. It was found that CXCR3 levels were boosted as a result of this treatment (Figure 7A), and that CTL activity was unaffected (N.B. IL-2 addition was also found to boost levels of CXCR3 positive cells, but this was not significant, and thus beads were used for the remainder of the mouse studies).
Figure 7.
Treatment of mouse CIK cells with CD3/CD28-coated beads results in increased CKR expression, enhanced trafficking and enhanced anti-tumour effect. (A) Levels of the CKR CXCR3 are increased after exposure to beads for 24 h in a day 14 culture (N.B. cellular cytotoxivity was unchanged, data not shown). (B) Transwell assay was used to follow movement of CIK cells (day 14, with or without bead treatment) towards lower wells containing recombinant IP-10 (Biolegend). Numbers of cells in the lower wells were determined by flow cytometry after 3 h. (C) Trafficking of bead-treated mCIK cells is significantly improved for in vivo delivery to subcutaneous MC38 tumours following tail-vein delivery of mCIK cells bound to NHS Cy5.5. CIK delivery to the tumour at 24 h after injection was determined by fluorescence imaging (using the FMT2500, Perkin Elmer) (n=4). Significantly greater CIK cell fluorescence was found in the tumour for CIK cell preparations that had been pretreated with CD3/CD25-coated beads (P=0.01). (D) Tumour volumes were also measured at the time of killing for these same groups (at day 7 post treatment), and an increased therapeutic effect was seen for bead-treated CIK cells (n=8, P=0.003).
The in vivo trafficking of anti-CD3/anti-CD28-coated bead-treated and untreated CIK cells was then examined after systemic delivery to tumour-bearing mice. It was determined that the use of the beads to boost CXCR3 levels also resulted in enhanced delivery of CIK cells to their tumour targets (Figure 7B). This also resulted in a small but significant increase in anti-tumour activity (Figure 7C).
Discussion
CIK cells represent an important model system for studying the trafficking of T cells to tumours as, unlike many vaccine induced T-cell populations, they efficiently traffic to both large and small solid tumours in mouse models(Baker et al, 2001; Thorne et al, 2006). A better understanding of how this trafficking is mediated will be important for establishing how to optimise CIK therapy, as well as how to enhance other immunotherapies such as adoptive T-cell transfer or cancer vaccines. However, such studies are complicated by the autologous nature of CIK cells, meaning that no immortalised cell line exists for study.
The importance of cell-signaling pathways induced by binding of chemokines to chemokine receptors in the directed trafficking of immune cell populations is well known (Lippitz, 2013), and thus it is likely that these pathways are involved in CIK cell trafficking, although there are only limited reports of CKR expression profiles on CIK cells, especially in a human setting.
Here, we initially incorporated multiple expansions from different human donors to try to identify specific CKR patterns in human CIK cell populations. This determined (i) that hCIK cells express an impressive number of different CKRs. This might be a necessary requirement for trafficking to multiple different tumour types that may produce different chemokine profiles, and (ii) that there is a high degree of variability in the relative numbers of cells positive for these different CKRs. This appears to be determined by the donor, as relative levels of CKRs did not vary significantly during the expansion process. The importance of differences in the particular chemokine receptor patterns remains to be established. More in-depth profiling of the chemokines produced by different tumours and how this correlates with chemokine receptor profiles on CIK cells and subsequent therapeutic effects will be needed to determine the clinical importance of different CKR expression; (iii) the level of CKR expression on hCIK cells peaks around day 14, and subsequently declines. This is reproducible among different donors and may be especially important, as CIK cells are usually infused back into a patient after about 28 days. This time was shown to correlate with maximum cytotoxic capability of the CIK cells, but it is clearly sub-optimal for trafficking. As a result, it is likely that CIK cell therapies could be tailored in the clinic, with, for example, earlier (day 14) expansions used when cell trafficking is more important (such as in combination studies where CIK cells may be used primarily as a delivery vehicle) and later (day 28) expansions used when cytolytic activity is critical (such as when treating leukaemia).
The importance of trafficking in the treatment of solid tumours was verified in mouse models. It was confirmed that mouse CIK cells also lost CKR surface expression over time in culture, and that loss of CKR reduced the trafficking of the cells to the tumour, leading to a reduction in therapeutic activity.
An alternative approach would be to re-stimulate older CIK cell expansions to boost CKR expression levels. This was attempted with a variety of different immune stimulants, with the most reliable results coming after exposure of older CIK cultures to anti-CD3/anti-CD28-coated beads. This led to an increase in the number of cells expressing many different CKRs within the CIK population, and also an increase in overall cytolytic capacity. As a result, not only could trafficking be improved at the times when cytolytic activity was peaking (as we have demonstrated in mouse tumour models), but also the variability between CKR levels seen between different hCIK preparations could be ‘normalised', leading to more consistent clinical results.
This approach would require only minor adjustment to the currently used clinical expansion protocols, and thus clinical testing would be simple, and appears justified based on these pre-clinical studies.
Acknowledgments
This work was supported directly by NIH grant R01 CA140215. In addition core facilities used in this work were supported by P30 CA047904-21S3.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- Belin LJ, Ady JW, Lewis C, Marano D, Gholami S, Mojica K, Eveno C, Longo V, Zanzonico PB, Chen NG, Szalay AA, Fong Y. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery. 2013;154:486–495. doi: 10.1016/j.surg.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchtner C, Emma DA, Manetta A, Gamboa G, Bernstein R, Liao SY. Characterization of a human ovarian carcinoma cell line: UCI 101. Gynecol Oncol. 1993;48:203–209. doi: 10.1006/gyno.1993.1034. [DOI] [PubMed] [Google Scholar]
- Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- Liu C, Suksanpaisan L, Chen YW, Russell SJ, PENG KW. Enhancing cytokine-induced killer cell therapy of multiple myeloma. Exp Hematol. 2013;41 (6:508–517. doi: 10.1016/j.exphem.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- Marin V, Dander E, Biagi E, Introna M, Fazio G, Biondi A, D'amico G. Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy. Exp Hematol. 2006;34:1219–1229. doi: 10.1016/j.exphem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Baker J, Beilhack A, Zeiser R, Olson JA, Sega EI, Karimi M, Negrin RS. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Melder RJ, Whiteside TL, Herberman RB, Jain RK. Preferential localization of human adherent lymphokine-activated killer cells in tumor microcirculation. J Natl Cancer Inst. 1991;83:433–437. doi: 10.1093/jnci/83.6.433. [DOI] [PubMed] [Google Scholar]
- Scheffold C, Kornacker M, Scheffold YC, Contag CH, Negrin RS. Visualization of effective tumor targeting by CD8+ natural killer T cells redirected with bispecific antibody F(ab')(2)HER2xCD3. Cancer Res. 2002;62:5785–5791. [PubMed] [Google Scholar]
- Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679. [PubMed] [Google Scholar]
- Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Zhao H, Fan Y, Li H, Yu J, Liu L, Cao S, Ren B, Yan F, Ren X. Immunotherapy with cytokine-induced killer cells as an adjuvant treatment for advanced gastric carcinoma: a retrospective study of 165 patients. Cancer Biother Radiopharm. 2013;28 (4:303–309. doi: 10.1089/cbr.2012.1306. [DOI] [PubMed] [Google Scholar]