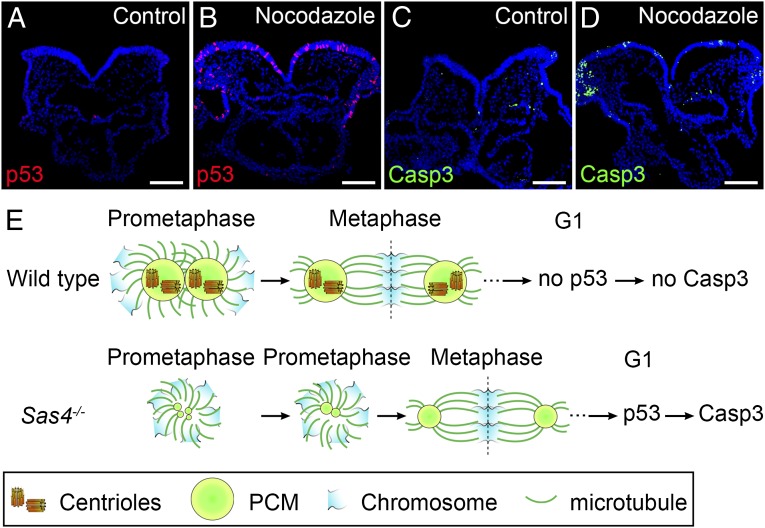
Fig. 7.
Prolonged prometaphase activates p53 and apoptosis in the wild-type mouse embryo. (A–D) Cross-sections of cultured wild-type E8.5 embryos treated with vehicle (DMSO) (A and C) or 1 µM nocodazole for 1 h (B and D) and harvested 4 h after nocodazole removal. Approximately 11% of the cells in nocodazole-treated embryos are p53+ (red) (B). The number of Casp3+ (green) cells also was elevated in nocodazole-treated embryos (D), indicating increased apoptotic cell death. (E) Model for the activation of p53 and apoptosis by acentriolar mitosis. (Upper) Mitotic cells in wild-type embryos use centrioles to recruit more PCM and form mature centrosomes that organize microtubules and establish the bipolar mitotic spindle during prometaphase. The chromosomes are aligned quickly and efficiently at the metaphase plate of a well-structured bipolar spindle. Mitosis proceeds normally, and p53 and Casp3 are not up-regulated in the daughter cells. (Lower) Mitotic cells in Sas4−/− embryos lack centrioles and rely primarily on a chromatin-based pathway to organize the mitotic spindle. The establishment of bipolar spindle poles is slower in the absence of centrioles, but acentriolar cells eventually assemble a bipolar spindle and align the chromosomes at the metaphase plate, followed by proper chromosome segregation. However, the daughter cells of the acentriolar division retain the memory of the prolonged prometaphase and up-regulate p53, which increases the probability of cell death (marked by Casp3 expression). (Scale bars: 100 µm.)