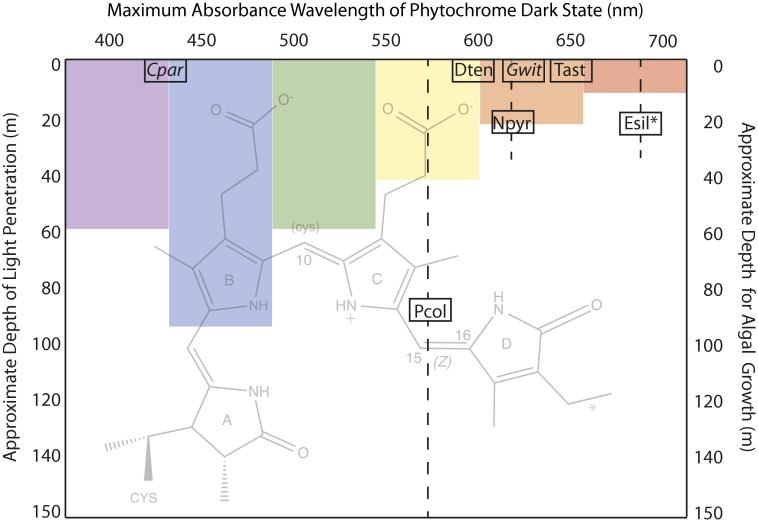
Fig. 1.
Eukaryotic algal phytochromes use phycocyanobilin (PCB) or PΦB and one- or two-cysteine photocycles to sense a wide range of the visible spectrum. The seven phytochromes described by Rockwell et al. in PNAS (2) are plotted according to the maximum absorbance wavelength of their dark state chromophore (x axis) and the approximate midpoint of the depth range (shown as a dashed line) at which they are found in either salt water (plain text) or fresh water (italics) (2). The protein is covalently attached to the A ring of the PCB chromophore (background image, with an asterisk indicating the position of a double bond in PΦB) via a thioether link and, for those examples that are underlined, via a second cysteine to C10 in the dark state only, interrupting the conjugated bond system and blue-shifting the absorbance maximum of the photoproduct. In all cases, photon absorbance causes a photoisomerization of the D ring around the C15=C16 bond. The seven phytochromes tested are encoded in the unicellular prasinophytes Tetraselmis astigmatica (Tast), Dolihomastix tenuilepis (Dten), Nephroselmis pyriformis (Npyr), and Prasinoderma coloniale (Pcol); in the filamentous multicellular brown alga Ectocarpus siliculosus (Esil); and in the fresh water microscopic Glaucophytes Cyanophora paradoxa (Cpar) and Gloeochaete wittrockiana (Gwit). As a schematic guide only, the approximate penetration depths at which 99% of light has been absorbed for various wavelengths in coastal waters is indicated (1).