Significance
The evolution and maintenance of diversity through cycles of past climate change have hinged largely on the availability of refugia. Geothermal refugia may have been particularly important for survival through past glaciations. Our spatial modeling of Antarctic biodiversity indicates that some terrestrial groups likely survived throughout intense glacial cycles on ice-free land or in sub-ice caves associated with areas of geothermal activity, from which recolonization of the rest of the continent took place. These results provide unexpected insights into the responses of various species to past climate change and the importance of geothermal regions in promoting biodiversity. Furthermore, they indicate the likely locations of biodiversity “hotspots” in Antarctica, suggesting a critical focus for future conservation efforts.
Keywords: dispersal, GIS, polar region, volcano, Last Glacial Maximum
Abstract
Climate change has played a critical role in the evolution and structure of Earth’s biodiversity. Geothermal activity, which can maintain ice-free terrain in glaciated regions, provides a tantalizing solution to the question of how diverse life can survive glaciations. No comprehensive assessment of this “geothermal glacial refugia” hypothesis has yet been undertaken, but Antarctica provides a unique setting for doing so. The continent has experienced repeated glaciations that most models indicate blanketed the continent in ice, yet many Antarctic species appear to have evolved in almost total isolation for millions of years, and hence must have persisted in situ throughout. How could terrestrial species have survived extreme glaciation events on the continent? Under a hypothesis of geothermal glacial refugia and subsequent recolonization of nongeothermal regions, we would expect to find greater contemporary diversity close to geothermal sites than in nongeothermal regions, and significant nestedness by distance of this diversity. We used spatial modeling approaches and the most comprehensive, validated terrestrial biodiversity dataset yet created for Antarctica to assess spatial patterns of diversity on the continent. Models clearly support our hypothesis, indicating that geothermally active regions have played a key role in structuring biodiversity patterns in Antarctica. These results provide critical insights into the evolutionary importance of geothermal refugia and the history of Antarctic species.
Climate change has played a fundamental role in the evolution and structure of Earth’s biodiversity (1, 2). Many contemporary diversity and distribution patterns, for example, appear to have been driven largely by the glacial and interglacial cycles of the Pleistocene, particularly latitudinal range changes with species moving toward glaciers that are receding (or away from those that are growing) (3, 4). Recent evidence indicates, however, that diverse life must have persisted throughout the Last Glacial Maximum (LGM) within areas thought to have been covered by large ice sheets (5–8).
Several disparate studies raise the possibility that geothermal areas might act as long-term refugia for a range of species under glacial conditions. These studies include the inferred persistence of a subterranean amphipod species in geothermally created refugia under the Icelandic ice cap (9), of a Patagonian crab in hot springs (10), of bryophytes on the isolated South Sandwich Islands of the maritime Antarctic (11), and of marine life during Snowball Earth glaciations in the Neoproterozoic (12). No comprehensive assessment of this “geothermal glacial refugia” hypothesis (11) has, however, yet been undertaken.
The isolated and heavily glaciated continent of Antarctica presents an ideal physical setting in which to test this hypothesis. A striking discrepancy exists between geological and glaciological evidence that Antarctica was heavily glaciated at the LGM [with ice extending offshore to the edge of the continental shelf for the West Antarctic Ice Sheet and at least to midshelf areas for much of the East Antarctic Ice Sheet (13)] and biological evidence indicating that many Antarctic terrestrial taxa have existed on the continent for millions of years (e.g., refs. 14–19). Indeed, it is becoming increasingly apparent that, with the exception of highly mobile marine birds and mammals, little natural colonization of Antarctica has occurred since the LGM (3). How did Antarctic terrestrial species [many of which are endemic (19–22)] that require an ice-free habitat, such as microarthropods, nematodes, and mosses, survive on what is thought to have been a continent almost completely covered in ice?
Geothermal sites may hold the answer. Numerous Antarctic volcanoes are currently active or have been active since and during the LGM, and these form three general clusters: the northern Antarctic Peninsula, Marie Byrd Land, and Victoria Land (23) (Fig. 1A). Ice-free terrain close to active craters, lower altitude ice-free geothermal ground (e.g., heated ground and ponds, steam fields, fumaroles), and ice caves formed by geothermal steam (24) could have existed throughout the Pleistocene (11), providing habitable environments that allowed Antarctic plants and invertebrates to survive on the continent. Recent geological estimates of ice thicknesses during the past 10 million years suggest that the ice cover was generally thinner than was previously thought, raising the likelihood of small, ice-free rocky patches (“nunataks”) being present (25, 26). However, although such nunataks could have harbored some life, many nunatak fauna are unique to such environments or to specific parts of the continent (27–29), and nunataks thus cannot explain the persistence of a wider range of Antarctic species, especially coastal species, throughout the LGM (30). Similarly, although the McMurdo Dry Valleys are known to have been partly ice-free at the LGM (31, 32), supporting some life, typically low moisture levels drastically limit diversity in this region (24, 25). Areas of greater biodiversity in Antarctica are usually thought to represent refugia of some kind that must have escaped the full consequences of various periods of glaciation, mostly as a consequence of typical geophysical features, such as permanently ice-free ridges or ablation valleys (33, 34). By contrast, little mention is made of the possibility of geothermal glacial refugia for anything but currently heated sites.
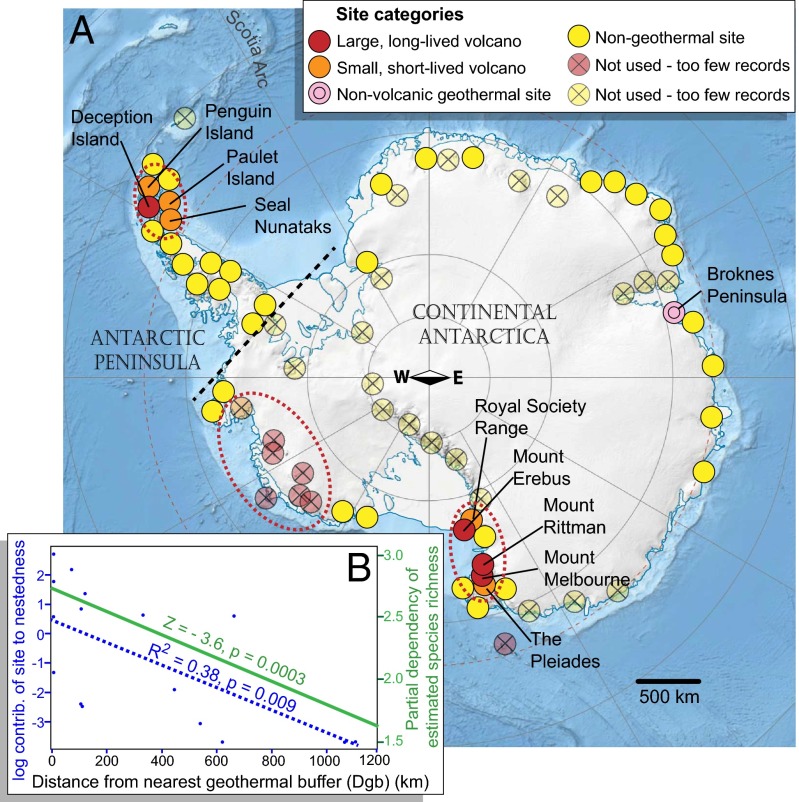
Fig. 1.
(A) Map of sites used in modeling analyses, classified as large volcanic (red), small volcanic (orange), nonvolcanic geothermal (pink), or nongeothermal (yellow). The circles show sampling units as used in the modeling analyses; these sites were areas with a radius of 100 km centered on an ice-free geothermal (e.g., volcano summit) or nongeothermal site, with species richness for each unit used as the modeling response variable [models tested effects of (i) a unit being geothermal or not and (ii) distance of nongeothermal units from the nearest geothermal]. The Antarctic Peninsula and “continental” Antarctica were analyzed separately; the division between these regions is indicated by a black dashed line. Broadly, there are three “clusters” of volcanoes in Antarctica, which are indicated here by dashed red ellipses. (B) Partial dependence plot shows the relationship of the estimated species richness (Sjack2) with distance from geothermal sites (green solid line and right y axis) and relationship of the log of the contribution of sites to nestedness (blue dashed line and left y axis) with increasing distance from the nearest geothermal site, for plants on the Antarctic Peninsula.
Here, through broad-scale spatial analysis of terrestrial biodiversity patterns in Antarctica, we directly test the geothermal refugia hypothesis. Nearly 39,000 spatially explicit occurrence records of 1,823 taxa south of 60° S have recently been compiled into the most comprehensive biodiversity database of Antarctic terrestrial taxa available to date (35). We used this dataset and spatial modeling approaches to assess diversity patterns in Antarctica in relation to the locations of geothermal areas. Postglacial population expansion away from LGM refugia is typically marked by a distinct reduction in diversity in recolonized vs. refugial regions (36–39) [including intraspecific genetic diversity (15, 31, 32)]. Under a hypothesis that geothermal refugia allowed persistence of Antarctic species throughout recent glacial periods, species richness is predicted to be highest close to geothermally active areas and lower in nonactive regions, with significant nestedness (40) of taxa from high- to low-richness areas, consistent with postglacial recolonization of more distant sites by a subset of the refugial taxa.
Results
Whether or not a site was geothermal was found to be an important predictor of its species richness [estimated for each site using the second-order jackknife estimator (41)], with the best models indicating higher species richness at geothermal vs. nongeothermal sites for both plants (mostly mosses) and fungi (mostly lichens, which represent symbioses of algae or Cyanobacteria and fungi) (Table 1 and Table S1). “Large geothermal site” was a significant predictor of plant species richness in the best model, whereas “small geothermal site” was a significant predictor in the best model of fungal species richness. When the data were analyzed on a regional basis, plant species richness showed the greatest effect, being influenced by both large and small geothermal sites on the Antarctic Peninsula and outside the Antarctic Peninsula (Fig. 1A and Table 1; note that there was only one “large” geothermal site on the Antarctic Peninsula vs. four elsewhere). However, the effect on fungal species richness was not discernible at the regional scale, with more conventional factors, such as area and altitude, being more important. Across all spatial scales of analyses (whole of Antarctica to regional), large or small geothermal sites did not appear in the best models of invertebrate species richness, although low numbers of records prevented confidence in these animal-only models.
Table 1.
Best models found to explain variance in estimated Sjack2 in relation to whether a site was geothermal or not, or to distance of a nongeothermal site from nearest geothermal site
| Region | Plants | AIC or DIC# (% exp) | Invertebrates | AIC or DIC# (% exp) | Fungi | AIC or DIC# (% exp) |
| Geothermal or not | ||||||
| Entire Antarctica | (+)Large geothermal sites***; ACBRRE; (+)log(NR)***; (+)AIF** | 132 (77%) | ACBRRE; (+)log(NR)***; (−)HASL** | 118# (74%) | (+)Small geothermal sites*; (+)log(NR)***; (−)HASL*; (−)RR#; s(lat,lon)*** | 339 (99%) |
| Antarctic Peninsula | (+)Large***, (+)small geothermal sites; ACBRRE; (+)log(NR)*** | 94 (91%) | ACBRRE; (+)log(NR)***; (−)HASL** | 10 (98%) | ACBRRE; (+)log(NR)***; (−)HASL* | 131# (89%) |
| Continental Antarctica | (+)Large***, (+)small geothermal sites*; ACBRRE; (+)log(NR)***; (−)HASL***; (−)RR* | 45 (88%) | ACBRRE; (−)RR***; (+)HASL*** | 11 (97%) | ACBRRE; (+)log(NR)***; (−)TDD***; (+)log(AIF)*; (+)RR**; (−)HASL*** | 66 (89%) |
| Distance from nearest geothermal site | ||||||
| Antarctic Peninsula | (−)Distance from geothermal***; ACBRRE; (+)log(NR)***; (−)AIF#; (−)RR#; (−)TDD | 32 (89%) | ACBRRE; (+)log(NR)***; HASL** | 10 (98%) | (−)Distance from geothermal*; ACBRRE; (+)log(NR)***; (+)AIF**; (−)HASL** | 36 (89%) |
| Continental Antarctica | (−)Distance from geothermal*; ACBRRE; (+)log(NR)***; (−)TDD*; (+)HASL#; (−)RR*** | 54 (85%) | (−)Distance from geothermal***; ACBRRE; (−)RR*** | 12 (89%) | (−)Distance from geothermal***; ACBRRE; (+)log(NR)***; (−)TDD*; (−)HASL#; (+)RR*** | 76 (86%) |
Geothermal sites were categorized as short-lived or long-lived. Boldface shows where geothermal factors were present in the best models for each analysis. Other predictors were as follows: ACBRRE, AIF (square kilometers), HASL, NR, RR, and TDD. s(lat,lon) (smoothed spatial covariate). Parameter details and data sources are provided in Table S7. Signs of model coefficients are shown in parentheses. Significance levels of predictors were assessed using Wald’s Z statistic and Bayesian p value (pMCMC) on the following scale: ***P < 0.001; **P < 0.01; *P < 0.05; #P < 0.08. No significance symbol indicates that the predictor was in the best model [by Akaike information criterion (AIC)] but not significant by the Wald test. A log term was added to predictors on the basis of partial residual plots. AIC or DIC# (% exp) provides estimates of the deviance explained by the best models. DIC, deviance information criterion; % exp, % deviance explained.
Distance to nearest geothermal site had a strong effect in models of plants, fungi, and invertebrates (Table 1 and Table S2). Regionally, a clear geothermal effect on species richness was seen on the Antarctic Peninsula, with distance to nearest geothermal site being a significant predictor in the best model of plant species richness (Fig. 1B) and a significant predictor in the best model of fungal species richness, but not for invertebrate richness. Distance to nearest geothermal site was also found to be an important driver of species richness for continental Antarctica (excluding the Antarctic Peninsula, indicating that the effect is not simply related to distance from the biodiverse islands of the Scotia Arc) for plants, fungi, and invertebrates.
Across all of the analyses, the best models typically explained a high proportion of the deviance (ca. >70%) (Table 1), indicating that the predictors included in the best models captured much of the variation in estimated richness for the groups at all of the scales examined. Similar outcomes, including the importance of geothermal sites, were found using observed species richness, although the predictors differed in some of the best models (Tables S3 and S4).
The Antarctic Peninsula nestedness analyses provided further insights into the diversity gradients. Using a metric based on nestedness overlap and decreasing fill (NODF) (40), nestedness was found to be significant, although not strong, along the species richness gradients for plants (NODF = 46.4), invertebrates (NODF = 43.0), and fungi (NODF = 37.3) (no evidence for the null hypothesis, Ho, P > 0.05 for all datasets). Similar results were found when the analyses used a “distance from nearest geothermal site” gradient, with all three taxonomic groups showing significant NODF values. The contribution of each site to the overall nestedness value decreased significantly with distance from closest geothermal site for plants (R2 = 0.38, P = 0.009; Fig. 1B) and fungi (R2 = 0.39, P = 0.008), but there was no clear relationship between these parameters for invertebrates (R2 = 0.17, nonsignificant). Nestedness analyses were conducted only on the Antarctic Peninsula because this region provided the best range of sampling units at nonadjacent, progressive distances from the geothermal sites, with relatively high numbers of records for most sites.
The Antarctic Conservation Biogeographic Region (35) in which each site was located, number of records (NR), and mean height above sea level (HASL) were the other most important predictors in the best models, with mean rugosity (RR) as a measure of habitat heterogeneity and area of ice-free land (AIF) also present in several models (Table 1). The general additive models (GAMs) with the smoothed spatial location covariate suggested that estimated species richness of the sites was spatially autocorrelated. However, the good fit of the generalized linear mixed effects models (GLMMs), as indicated by model diagnostics, suggested that with most of the datasets, the Antarctic Conservation Biogeographic Regions as a random effect (ACBRRE), together with the other covariates, effectively explained much of the variation in species richness and likely accounted for much of the spatial autocorrelation, without the potential concerns of overfitting inherent in the GAMs. Simultaneous autoregressive models and Poisson models with a spatial autocovariate were also fitted to the data but, like most of the GAMs, did not fit the data as well as the GLMMs.
Discussion
Across Antarctica, estimated species richness is, in the majority of cases, higher in or close to geothermal regions compared with nongeothermal regions, and assemblages further away from geothermal sites form subsets of those in the geothermal locations along the Antarctic Peninsula. This pattern of diversity is entirely unexpected for the continent. Past work has typically focused on species-energy or latitudinal gradient relationships, frequently noting that they break down for the continent or that local microclimatic conditions determine diversity patterns (42–46). Importantly, because the sample units used here have a 100-km radius around either geothermal or nongeothermal sites, the outcomes do not reflect the current effects of heated ground at the geothermal sites. At least in the polar regions, heated ground tends to result in elevated vegetation cover and richness (11, 47, 48), whereas at sites elsewhere, cover might increase but richness can decline with adverse temperature and soil properties (e.g., refs. 49, 50). Furthermore, by including degree days, HASL, RR, Antarctic Conservation Biogeographic Region, and NR in the analyses, the potential effects of larger scale species-energy relationships, habitat heterogeneity, or similar spatially explicit patterns, as well as the potential effects of the proximity of sites to research stations (potentially better surveyed regions than elsewhere), were accounted for. These other factors cannot be substituted as an explanation for the significance of geothermal areas in the analytical outcomes. Our results thus provide support for the hypothesis that geothermally heated terrain plays a significant role in structuring broad-scale patterns in Antarctic diversity by providing glacial refugia.
The results also indicate different abilities among broad taxonomic groups to capitalize on geothermal habitats and disperse away from these refugia. Plants showed particularly strong patterns, with higher species richness on and around large and small geothermal sites than in nongeothermal regions. The vast majority of plant records are of mosses (Bryophyta), so although some other groups were included in our analyses, we focus our discussion on mosses. Many mosses have lightweight spores, facilitating aerial dispersal, and propagules from several moss species have been found in air traps in Antarctica (51). Although it has therefore long been thought that many mosses must have dispersed recently to Antarctica (46), new molecular, taxonomic, and subfossil evidence challenges this assumption, indicating that some species have persisted on the continent throughout Pleistocene glacial cycles in refugia (52, 53). Furthermore, several mosses are endemic to Antarctica, and thus are most likely to have survived glaciations in situ (54). Geothermal regions have been reported to support some moss species more readily than nongeothermal regions in Antarctica (55). The fact that mosses were found to be significantly more species-rich at geothermal sites than at nongeothermal sites (and, on the Antarctic Peninsula, that diversity was found to decrease in a nested manner with increasing distance from such sites) indicates that, despite their strong dispersal ability, only a subset of moss species have been able to colonize and persist in nongeothermal regions. Site-hopping may, however, occur readily for some Antarctic mosses. For example, Skotnicki et al. (56) found close phylogenetic relationships among samples of the moss Campylopus pyriformis from two populations near the summit of Mount Melbourne and a sample from Mount Erebus, more than 350 km away. Broadly, the evidence suggests that geothermal regions most likely acted as glacial refugia for nonvascular plants in Antarctica, with aerial transfer maintaining diversity among patches of ice-free terrain and allowing some, but probably rare and stochastic, long-distance dispersal for recolonization of the rest of the continent.
Geothermal regions had a clear effect on fungal species richness, with decreasing diversity away from active sites, although patterns were somewhat less striking than for plants. Most (although not all) fungal species records in the dataset were of lichen-forming fungi (Ascomycota). Antarctic lichens may be extremely long-lived (several hundreds of years) and slow growing, particularly at high altitudes and inland (57). Perhaps most significantly, some can survive temperatures as low as −196 °C (58), rehydrate following desiccation, and disperse via aerial transport of spores and other propagules (59). Therefore, they may also have been able to survive glacial cycles on rock outcrops protruding through the expanded ice sheets. For these hardy taxa, geothermal regions, although clearly important, may not have been as critical as they appear to have been for plants.
Dispersal away from geothermal refugia could be predicted to be more difficult for many animal taxa, which lack airborne propagules, than for mosses or lichens. Thus, these taxa might be expected to show more striking diversity gradients in relation to the locations of refugia than the more readily dispersed mosses and lichens. Such patterns were observed in the regional continental analyses but not on the Antarctic Peninsula, perhaps reflecting the fact that the faunal analyses were limited by smaller datasets than were available for plants and fungi (Table S5). Based on our evidence that geothermal refugia have been important for mosses and lichens, such refugia should have been at least as important for the Antarctic invertebrate taxa, many of which rely on or are strongly associated with vegetated habitats (54, 55). Whereas poor sampling is the most parsimonious explanation for this pattern, other factors might also be important. Most plausible among these factors is a larger influence of survival on LGM nunataks on invertebrates than on other taxa (19), although, as stated above, contemporary nunataks do not host the same suite of invertebrates as found in coastal regions (30). Genetic research on intraspecific spatial patterns of diversity could shed light on whether invertebrates have recolonized much of the continent primarily from geothermal refugia or whether nunataks have played a larger role.
The current results raise the possibility that geothermal glacial refugia may also have played a fundamental but underappreciated role in maintaining biodiversity throughout glacial periods elsewhere. Indeed, the theory that major glaciated regions such as northern Europe and northern North America were completely ice covered at the LGM has been challenged in recent years by several biological studies. Although many taxa do show evidence of having been extirpated from large areas, recolonizing poleward as the ice retreated (31, 34), some species appear to have survived within the glaciated regions in small refugia (often called “cryptic” refugia or “microrefugia”) (5–8). In many cases, the existence of these refugia has been inferred from phylogeographic data (35, 38), but their precise locations and causes have not been identified. Several of these refugia may have been geothermal. Microrefugia have, for example, been proposed for Norway (8), where considerable geothermal heat is generated from radiogenic decay of bedrock (60). For Iceland, the debate has swung between arguments that the biota could only have colonized following the post-LGM recession of the ice cap (61) and recent evidence that some species survived in situ in sub-ice geothermal refugia (40, 41). Likewise, in South America, hot springs appear to have maintained ice-free lakes within glaciated regions, allowing species such as freshwater crabs to survive (10). Our results suggest that geothermal warming of parts of Antarctica has not only shaped patterns of diversity but has been an integral part of maintaining life on the continent throughout glacial and interglacial cycles.
Perhaps because of their small area, high temperatures, mineralization, and reputation for constant habitat change, geothermal areas have rarely been identified as potential refugia (49, 62). Nonetheless, contemporary active regions can harbor a diverse biota, even soon after eruption (63, 64). Despite volcanic geothermal regions typically having greater pH and mineralization of soils than nongeothermal regions, many species found in such areas are more broadly distributed and appear simply to capitalize on the warming and water availability provided by geothermal activity (48, 65).
In conclusion, our broad-scale spatial analyses provide support for the intriguing hypothesis that geothermal areas have played a critical role in the maintenance and structure of diversity across Antarctica. Geothermal areas may also provide a solution to the mystery of how life survived widespread ice formation elsewhere during Pleistocene glacial maxima and previously (12), emphasizing their broader significance in terrestrial as well as marine (66) systems. Furthermore, our findings suggest that geothermal regions may represent diversity “hotspots” in Antarctica, providing a focus for conservation efforts. In so doing, they highlight the urgent need for increased sampling to identify biodiversity patterns in underrepresented areas, such as Marie Byrd Land (west of the Ross Sea), particularly given the growing evidence of negative impacts on Antarctic biodiversity from climate change and anthropogenic disturbance (67).
Methods
Our analyses used circular areas with a radius of 100 km (centered on either geothermal or nongeothermal sites) as sampling units with which to assess the most important factors underpinning spatial patterns of species richness. A total of 33 nongeothermal units and 10 geothermal units were included in analyses. Models assessed species richness patterns based on (i) whether a unit was geothermal or nongeothermal and (ii) the distance of a nongeothermal unit from the nearest geothermal unit (testing whether species richness declines away from geothermal regions).
Sixteen Antarctic volcanoes are known to have been active since the LGM (Fig. 1A). Ten of these sites are large, long-lived polygenetic centers of volcanic activity (23), potentially capable of maintaining ice-free terrain through geothermal heating even during periods of inactivity, because they each have a crustal magma chamber that slowly cools over many tens of thousands of years, providing heat to the ground above as fumaroles or steam fields and as summit ice caves. Many of these large volcanic centers have existed for hundreds of thousands of years (23), and numerous studies have demonstrated that several were almost certainly active during or close to the LGM [e.g., Mount Erebus, Mount Melbourne, The Pleiades, Mount Berlin, possibly Mount Takahe (68–70)]. We classified these large centers as “long-lived” in our analyses, whereas all other volcanoes (Table S6) lack large magma chambers (23) and are individually small and monogenetic (i.e., underwent only a single brief eruptive period), and so were classed as “short-lived.” Antarctica contains many other volcanoes (23), but because any biodiversity impact of those volcanoes that have not been active since the LGM would have been erased during the LGM or before, these long-dormant volcanoes were not included as geothermal sites in our analyses.
Geothermal heating in Antarctica is also known from the Broknes Peninsula in the Larsemann Hills region which, despite being nonvolcanic, has relatively warm Cambrian rock heated through radiogenic decay (71), and which lacustrine sedimentary (72) and microfossil (73) evidence suggests may have been an unusual, ice-free refugium at the LGM. Because this radiogenic decay will have been ongoing for millennia, Broknes Peninsula was classed as a long-lived geothermal region in our analyses.
In addition to the 10 long-lived and the six short-lived geothermal sites, we selected as many randomly placed nongeothermal sites as possible to cover the available biodiversity data, with each centered on ice-free terrain. Sites that were poorly represented by sampling effort (fewer than 10 species records) were, however, excluded from analyses, because these sites would be unable to contribute meaningful information. Likewise, because all geothermal regions were close to the coast, comparing their species richness with that of inland regions would not be meaningful; thus, sites that were more than 200 km inland from summer open water (e.g., ice-free alpine terrain on the Transantarctic Mountains) were excluded. Our final analyses thus included 33 nongeothermal sites, five long-lived geothermal sites (Deception Island, Mount Erebus, Mount Melbourne, Mount Rittman, and the Broknes Peninsula in the Larsemann Hills), and five short-lived geothermal sites (Paulet Island, Penguin Island, Seal Nunataks, The Pleiades, and the Royal Society Range volcanoes) (Fig. 1A and Table S6).
With the exception of wind-dispersed taxa, such as mosses and lichens, most Antarctic terrestrial species are likely to be relatively poor dispersers that rely on rare, chance events, such as transport via birds (30), to colonize distant territory (e.g., greater than tens of kilometers). Geothermal heating from volcanic activity is generally limited to within a few kilometers of the summit crater or caldera. We therefore considered a buffer zone around each geothermal site of 100 km (radius) to be adequate to overcome the specific effect of current geothermal activity, incorporating both the potential glacial refuge and areas within the unassisted dispersal range of most taxa.
We used the Antarctic biodiversity dataset compiled by Terauds et al. (35), comprising 38,854 validated records across more than 30 phyla, in our spatial modeling analyses. These records were originally obtained through access to the Antarctic Biodiversity Database (http://data.aad.gov.au/aadc/biodiversity/). To refine the dataset for the current analyses, marine and sub-Antarctic records were first excluded and the remaining records were checked for spatially explicit data and taxonomic reliability. Manifold Professional v8.0 geographic information system software (Manifold Software Ltd.) was used to create separate spatial layers of 12 “collections” that were identified as containing useful data, and these collections were compiled into a single dataset for the analyses.
Because of the problems associated with using observed species richness in analyses of richness variation (74), we used estimated species richness determined by Jackknife 2 estimator (Sjack2) as an approximate measure of “biodiversity” at each site, and thus as the response variable in all models (excluding the nestedness analyses). We estimated richness for each site using the second-order jackknife estimator (41, 74) implemented in the R-package fossil::jack2. All analyses were also undertaken using observed species richness (Sobs) as the response variable.
We analyzed data on a regional basis, with sites on the Antarctic Peninsula separated from the rest of the continent. This separation was considered necessary because the distinction between biodiversity on the Antarctic Peninsula and biodiversity in continental areas has long been recognized (35, 42). For broad “geothermal vs. nongeothermal” analyses (see below), data were also analyzed for both regions together, but for the “distance from nearest geothermal,” models were only fitted on a regional basis because doing so was considered more appropriate to assess gradients. The data were also analyzed by taxonomic group, with three categories: “plants” (almost 90% of which were Bryophyta, with the remainder consisting of liverworts and terrestrial algae), “invertebrates” (80% of which were arthropods, with nematodes and tardigrades dominating the remainder), and “fungi” (96% lichen-forming fungi from the phylum Ascomycota). Microbial records were not sufficiently abundant and had limited distribution, so they were not included in the analyses. The numbers of data points varied considerably among sites but were spread relatively evenly across the two regions, with the exception of invertebrate records, which were considerably greater on the Antarctic Peninsula (Table S5).
We used a suite of modeling techniques to find the best predictors of species richness in each of the datasets. Predictors included a range of continuous and categorical sampling, climatic, landscape, and geothermal variables (a full description of parameter types and data sources is provided in Table S7). The Antarctic Conservation Biogeographic Regions [“bioregion” (35)] were incorporated in separate models as random effects (ACBRRE); temperature was represented by annual cumulative degree days with a −5 °C threshold (TDD), and altitude was calculated as the mean HASL from a 200-m digital elevation model. RR was also calculated from the digital elevation model and was included as a measure of habitat heterogeneity, given the significance of the latter as a factor influencing richness variation (75). Incorporating degree days allowed us to account for latitudinal effects (which were expected to be particularly strong for the Antarctic Peninsula), including species richness/temperature relationships as might be predicted under the metabolic theory of ecology (76). The NR from each site was used to account for sampling biases (e.g., proximity of sampling unit to research bases), and AIF was used to account for variation in available habitat. Geothermally related parameters included distance to nearest geothermal site and categorical variables indicating if a geothermal site was small or large.
Estimated species richness typically followed a right-skewed distribution; therefore, the Poisson distribution was considered an appropriate starting point for most models. For each dataset, simple generalized linear models [GLMs; R package stats::glm (77)] were first fitted using the Antarctic Conservation Biogeographic Regions as a fixed effect and a Poisson distribution with a log-link. In most cases, comparisons of the residual deviance with the residual degrees of freedom indicated that the data were overdispersed. Partial residual plots of the fitted models were used to clarify the relationship between R and each predictor. In several cases, these plots indicated an exponential relationship between R and NR and/or AIF. In these cases, a log term was added to these predictors (Table 1). Next, a GLMM was fitted using the R-package lme4:glmer (78), using a Gaussian–Hermite approximation to the log-likelihood and a Poisson distribution with a log link. In all cases, these models fitted the data much better than the fixed effects models, likely attributable to the inclusion of the random effect term reducing the overdispersion. Model diagnostics in the form of qq-plots, plots of residuals vs. fitted values, and AICs were also used to assess the fit of these models. If the diagnostic plots suggested a significant departure from linearity or heteroscedasticity of variance, other GLMMs were fitted, first using a negative binomial distribution [R package glmmADMB:glmmadmb (79)]; then, if there was no improvement, a Bayesian framework was used to fit Poisson-distributed GLMMs with Markov Chain Monte Carlo (MCMC) methods [R package MCMCglmm:MCMCglmm (80)]. There were no cases where models with a negative binomial distribution fitted better than the Poisson models with a Gaussian–Hermite approximation; thus, in the cases where the fit of the latter models was questionable, MCMC-based models were taken as the best models. However, in almost all cases, the best MCMC models returned similar predictors to the best Poisson models using the Gaussian–Hermite approximation. GAMs were also fitted, where the ACBRRE term was replaced with a bivariate smooth location term [s(lon,lat)] using the R-package mgcv::gam (81). These models needed larger sample sizes to converge, so they were only implemented using data from the whole of Antarctica. Simultaneous autoregressive models implemented in the R-package spdep::spautolm and Poisson GLMs using a spatial autocovariate generated using spdep::autocov_dist (82) were also examined.
The most parsimonious models within each model set (and dataset) were selected using AICs (83) for Gaussian–Hermite GLMMs, GAMs, and GLMs and using DICs (84) for the MCMC-based models. The Wald Z statistic was used as an indicator of parameter importance in the Gaussian–Hermite models. For the MCMC-based models, the pMCMC value was used to indicate parameter importance.
On the Antarctic Peninsula, we supplemented our modeling analyses with nestedness analyses using the NODF nestedness measure and associated software (40). We ran the analyses for each of the taxonomic groups using sites on the Antarctic Peninsula, with each matrix sorted by species richness and by distance to nearest geothermal site. The significance of the NODF metric was tested against the distribution of nestedness scores obtained from 500 to 1,000 null model matrices (depending on the size of the dataset), with the null model constructed using the proportional row and proportional column constraints [as described by Ulrich and Gotelli (85)]. We used the DeltaSic measure provided by the NODF software to quantify the contribution of each site to the overall degree of nestedness.
Supplementary Material
Acknowledgments
We thank Thomas van Boeckel, Bruno Danis, Chris Carson, Alessio Mortelliti, and Craig Cary for discussions; a wide range of researchers for biological records that contributed to the biodiversity database used in this research; and two anonymous reviewers for their insightful comments. The Australian Antarctic Data Centre provided the Radarsat Antarctic Mapping Project digital elevation model, Simon Wotherspoon provided statistical advice, and Ben Raymond helped generate the RR data. Research funds were provided by Australian Research Council Discovery Early Career Research Award DE140101715 (to C.I.F.), and A.T. was supported by the Australian Antarctic Division and the Scientific Committee on Antarctic Research (SCAR) State of the Antarctic Ecosystem (AntEco) Science Program. P.C. is supported by Natural Environment Research Council core funding to the British Antarctic Survey programs “Ecosystems” and “Environmental Change and Evolution.” This paper contributes to the SCAR AntEco research program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5452.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321437111/-/DCSupplemental.
References
- 1.Romdal TS, Araújo MB, Rahbek C. Life on a tropical planet: Niche conservatism and the global diversity gradient. Glob Ecol Biogeogr. 2013;22(3):344–350. [Google Scholar]
- 2.Dynesius M, Jansson R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc Natl Acad Sci USA. 2000;97(16):9115–9120. doi: 10.1073/pnas.97.16.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser CI, Nikula R, Ruzzante DE, Waters JM. Poleward bound: Biological impacts of Southern Hemisphere glaciation. Trends Ecol Evol. 2012;27(8):462–471. doi: 10.1016/j.tree.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci. 2004;359(1442):183–195, discussion 195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provan J, Bennett KD. Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol. 2008;23(10):564–571. doi: 10.1016/j.tree.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Maggs CA, et al. Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology. 2008;89(11) Suppl:S108–S122. doi: 10.1890/08-0257.1. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JR, Lister AM, Barnes I, Dalen L. Refugia revisited: Individualistic responses of species in space and time. Proc R Soc Biol Sci Ser B. 2010;277(1682):661–671. doi: 10.1098/rspb.2009.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart JR, Lister AM. Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol. 2001;16(11):608–613. [Google Scholar]
- 9.Kornobis E, Pálsson S, Kristjánsson BK, Svavarsson J. Molecular evidence of the survival of subterranean amphipods (Arthropoda) during Ice Age underneath glaciers in Iceland. Mol Ecol. 2010;19(12):2516–2530. doi: 10.1111/j.1365-294X.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu JW, Pérez-Losada M, Jara CG, Crandall KA. Pleistocene glaciation leaves deep signature on the freshwater crab Aegla alacalufi in Chilean Patagonia. Mol Ecol. 2009;18(5):904–918. doi: 10.1111/j.1365-294X.2008.04070.x. [DOI] [PubMed] [Google Scholar]
- 11.Convey P, Lewis Smith RI. Geothermal bryophyte habitats in the South Sandwich Islands, maritime Antarctic. J Veg Sci. 2006;17(4):529–538. [Google Scholar]
- 12.Schrag DP, Hoffman PF. Life, geology and snowball Earth. Nature. 2001;409(6818):306. doi: 10.1038/35053170. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JB, Shipp SS, Lowe AL, Wellner JS, Mosola AB. The Antarctic Ice Sheet during the Last Glacial Maximum and its subsequent retreat history: A review. Quat Sci Rev. 2002;21(1–3):49–70. [Google Scholar]
- 14.McGaughran A, Stevens MI, Holland BR. Biogeography of circum-Antarctic springtails. Mol Phylogenet Evol. 2010;57(1):48–58. doi: 10.1016/j.ympev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.De Wever A, et al. Hidden levels of phylodiversity in Antarctic green algae: Further evidence for the existence of glacial refugia. Proc R Soc Biol Sci Ser B. 2009;276(1673):3591–3599. doi: 10.1098/rspb.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Convey P, et al. Exploring biological constraints on the glacial history of Antarctica. Quat Sci Rev. 2009;28(27-28):3035–3048. [Google Scholar]
- 17.Allegrucci G, Carchini G, Todisco V, Convey P, Sbordoni V. A molecular phylogeny of Antarctic Chironomidae and its implications for biogeographical history. Polar Biol. 2006;29(4):320–326. [Google Scholar]
- 18.Mortimer E, et al. Mite dispersal among the Southern Ocean Islands and Antarctica before the last glacial maximum. Proc R Soc Biol Sci Ser B. 2011;278(1709):1247–1255. doi: 10.1098/rspb.2010.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugh PJA, Convey P. Surviving out in the cold: Antarctic endemic invertebrates and their refugia. J Biogeogr. 2008;35(12):2176–2186. [Google Scholar]
- 20.Bahl J, et al. Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nat Commun. 2011;2:163. doi: 10.1038/ncomms1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyverman W, et al. Evidence for widespread endemism among Antarctic micro-organisms. Polar Sci. 2010;4(2):103–113. [Google Scholar]
- 22.Øvstedal DO, Lewis Smith RI. Lichens of Antarctica and South Georgia: A Guide to their Identification and Ecology. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 23.LeMasurier WE. In: Volcanoes of the Antarctic Plate and Southern Oceans. Thomson JW, editor. Vol 48. Washington, DC: American Geophysical Union; 1990. [Google Scholar]
- 24.Giggenbach WF. Geothermal ice caves on Mt Erebus, Ross Island, Antarctica. New Zealand Journal of Geology and Geophysics. 1976;19(3):365–372. [Google Scholar]
- 25.Smellie JL, Haywood AM, Hillenbrand C-D, Lunt DJ, Valdes PJ. Nature of the Antarctic Peninsula Ice Sheet during the Pliocene: Geological evidence and modelling results compared. Earth Sci Rev. 2009;94(1–4):79–94. [Google Scholar]
- 26.Smellie JL, Rocchi S, Gemelli M, Di Vincenzo G, Armineti P. A thin predominantly cold-based Late Miocene East Antarctic ice sheet inferred from glaciovolcanic sequences in northern Victoria Land, Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;307(1-4):129–149. [Google Scholar]
- 27.Convey P, McInnes SJ. Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology. 2005;86(2):519–527. [Google Scholar]
- 28.Marshall DJ, Coetzee L. Historical biogeography and ecology of a Continental Antarctic mite genus, Maudheimia (Acari, Oribatida): Evidence for a Gondwanan origin and Pliocene-Pleistocene speciation. Zool J Linn Soc. 2000;129(1):111–128. [Google Scholar]
- 29.Sohlenius B, Boström S, Ingemar Jönsson K. Occurrence of nematodes, tardigrades and rotifers on ice-free areas in East Antarctica. Pedobiologia (Jena) 2004;48(4):395–408. [Google Scholar]
- 30.Convey P, et al. Antarctic terrestrial life—Challenging the history of the frozen continent? Biol Rev Camb Philos Soc. 2008;83(2):103–117. doi: 10.1111/j.1469-185X.2008.00034.x. [DOI] [PubMed] [Google Scholar]
- 31.Stevens MI, Hogg ID. Long-term isolation and recent range expansion from glacial refugia revealed for the endemic springtail Gomphiocephalus hodgsoni from Victoria Land, Antarctica. Mol Ecol. 2003;12(9):2357–2369. doi: 10.1046/j.1365-294x.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- 32.Hall BL, Denton GH, Overturf B. Glacial Lake Wright, a high-level Antarctic lake during the LGM and early Holocene. Antarct Sci. 2001;13(1):53–60. [Google Scholar]
- 33.Green TGA, Sancho LG, Türk R, Seppelt RD, Hogg ID. High diversity of lichens at 84°S, Queen Maud Mountains, suggests preglacial survival of species in the Ross Sea region, Antarctica. Polar Biol. 2011;34(8):1211–1220. [Google Scholar]
- 34.Smith RIL. Bryophyte oases in ablation valleys on Alexander Island, Antarctica. Bryologist. 1988;91(1):45–50. [Google Scholar]
- 35.Terauds A, et al. Conservation biogeography of the Antarctic. Divers Distrib. 2012;18(7):726–741. [Google Scholar]
- 36.Svenning J-C, Skov F. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol Lett. 2007;10(6):453–460. doi: 10.1111/j.1461-0248.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- 37.Svenning J-C, Skov F. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob Ecol Biogeogr. 2007;16(2):234–245. [Google Scholar]
- 38.Keppel G, et al. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob Ecol Biogeogr. 2012;21(4):393–404. [Google Scholar]
- 39.Lawes MJ, Eeley HAC, Findlay NJ, Forbes D. Resilient forest faunal communities in South Africa: A legacy of palaeoclimatic change and extinction filtering? J Biogeogr. 2007;34(7):1246–1264. [Google Scholar]
- 40.Almeida-Neto M, Guimarães P, Guimarães PR, Loyola RD, Ulrich W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos. 2008;117(8):1227–1239. [Google Scholar]
- 41.Hortal J, Borges PAV, Gaspar C. Evaluating the performance of species richness estimators: Sensitivity to sample grain size. J Anim Ecol. 2006;75(1):274–287. doi: 10.1111/j.1365-2656.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 42.Chown SL, Convey P. Spatial and temporal variability across life’s hierarchies in the terrestrial Antarctic. Philos Trans R Soc Lond B Biol Sci. 2007;362(1488):2307–2331. doi: 10.1098/rstb.2006.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cary SC, McDonald IR, Barrett JE, Cowan DA. On the rocks: The microbiology of Antarctic Dry Valley soils. Nat Rev Microbiol. 2010;8(2):129–138. doi: 10.1038/nrmicro2281. [DOI] [PubMed] [Google Scholar]
- 44.Casanovas P, Lynch HJ, Fagan WF. Multi-scale patterns of moss and lichen richness on the Antarctic Peninsula. Ecography. 2013;36(2):209–219. [Google Scholar]
- 45.Green TGA, Sancho L, Pintado A, Schroeter B. Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol. 2011;34(11):1643–1656. [Google Scholar]
- 46.Peat HJ, Clarke A, Convey P. Diversity and biogeography of the Antarctic flora. J Biogeogr. 2007;34(1):132–146. [Google Scholar]
- 47.Smith RIL. Colonization and recovery by cryptogams following recent volcanic activity on Deception Island, South Shetland Islands. British Antarctic Survey Bulletin. 1984;62:25–51. [Google Scholar]
- 48.Convey P, Smith RIL, Hodgson DA, Peat HJ. The flora of the South Sandwich Islands, with particular reference to the influence of geothermal heating. J Biogeogr. 2000;27(6):1279–1295. [Google Scholar]
- 49.Burns B. Vegetation change along a geothermal stress gradient at the Te Kopia steamfield. Journal of the Royal Society of New Zealand. 1997;27(2):279–293. [Google Scholar]
- 50.Chiarucci A, Calderisi M, Casini F, Bonini I. Vegetation at the limits for vegetation: Vascular plants, bryophytes and lichens in a geothermal field. Folia Geobot. 2008;43(1):19–33. [Google Scholar]
- 51.Marshall WA, Convey P. Dispersal of moss propagules on Signy Island, maritime Antarctic. Polar Biol. 1997;18(6):376–383. [Google Scholar]
- 52.Hills SFK, Stevens MI, Gemmill CEC. Molecular support for Pleistocene persistence of the continental Antarctic moss Bryum argenteum. Antarct Sci. 2010;22(6):721–726. [Google Scholar]
- 53.Singh SM, Ochyra R, Pednekar SM, Asthana R, Ravindra R. A Holocene moss species preserved in lake sediment core and the present moss diversity at Schirmacher Oasis, Antarctica. Antarct Sci. 2012;24(04):353–358. [Google Scholar]
- 54.Cannone N, Convey P, Guglielmin M. Diversity trends of bryophytes in continental Antarctica. Polar Biol. 2013;36(2):259–271. [Google Scholar]
- 55.Skotnicki M, Bargagli R, Ninham J. Genetic diversity in the moss Pohlia nutans on geothermal ground of Mount Rittmann, Victoria Land, Antarctica. Polar Biol. 2002;25(10):771–777. [Google Scholar]
- 56.Skotnicki ML, Selkirk PM, Broady P, Adam KD, Ninham JA. Dispersal of the moss Campylopus pyriformis on geothermal ground near the summits of Mount Erebus and Mount Melbourne, Victoria Land, Antarctica. Antarct Sci. 2001;13(3):280–285. [Google Scholar]
- 57.Lindsay DC. The role of lichens in Antarctic ecosystems. Bryologist. 1978;81(2):268–276. [Google Scholar]
- 58.Lange OL, Kappen L. Photosynthesis of lichens from Antarctica. Antarct Res Ser. 1972;20:83–95. [Google Scholar]
- 59.Kappen L. Some aspects of the great success of lichens in Antarctica. Antarct Sci. 2000;12(03):314–324. [Google Scholar]
- 60.Slagstad T. Radiogenic heat production of Archaean to Permian geological provinces in Norway. Norwegian Journal of Geology. 2008;88(3):149–166. [Google Scholar]
- 61.Buckland P, Dugmore A. ‘If this is a refugium, why are my feet so bloody cold?’ The origins of the Icelandic biota in the light of recent research. In: Maizels J, Caseldine C, editors. Environmental Change in Iceland: Past and Present, Glaciology and Quaternary Geology. Vol 7. Dordrecht, The Netherlands: Springer; 1991. pp. 107–125. [Google Scholar]
- 62.Stout RG, Al-Niemi TS. Heat-tolerant flowering plants of active geothermal areas in Yellowstone National Park. Ann Bot (Lond) 2002;90(2):259–267. doi: 10.1093/aob/mcf174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashmole NP, Oromi P, Ashmole MJ, Martin JL. Primary faunal succession in volcanic terrain—Lava and cave studies on the Canary Islands. Biol J Linn Soc Lond. 1992;46(1-2):207–234. [Google Scholar]
- 64.Thornton IWB, et al. Colonization of an island volcano, Long Island, Papua New Guinea, and an emergent island, Motmot, in its caldera lake. VII. Overview and discussion. J Biogeogr. 2001;28(11-12):1389–1408. [Google Scholar]
- 65.Boothroyd IKG. Ecological characteristics and management of geothermal systems of the Taupo Volcanic Zone, New Zealand. Geothermics. 2009;38(1):200–209. [Google Scholar]
- 66.Van Dover CL, German CR, Speer KG, Parson LM, Vrijenhoek RC. Evolution and biogeography of deep-sea vent and seep invertebrates. Science. 2002;295(5558):1253–1257. doi: 10.1126/science.1067361. [DOI] [PubMed] [Google Scholar]
- 67.Chown SL, et al. Conservation. Challenges to the future conservation of the Antarctic. Science. 2012;337(6091):158–159. doi: 10.1126/science.1222821. [DOI] [PubMed] [Google Scholar]
- 68.Licht KJ, Dunbar NW, Andrews JT, Jennings AE. Distinguishing subglacial till and glacial marine diamictons in the western Ross Sea, Antarctica: Implications for a last glacial maximum grounding line. Geol Soc Am Bull. 1999;111(1):91–103. [Google Scholar]
- 69.Kelly PJ, Dunbar NW, Kyle PR, McIntosh WC. Refinement of the late Quaternary geologic history of Erebus volcano, Antarctica using 40Ar/39Ar and 36Cl age determinations. J Volcanol Geotherm Res. 2008;177(3):569–577. [Google Scholar]
- 70.Dunbar NW, McIntosh WC, Esser RP. Physical setting and tephrochronology of the summit caldera ice record at Mount Moulton, West Antarctica. Geol Soc Am Bull. 2008;120(7-8):796–812. [Google Scholar]
- 71.Carson CJ, Pittard M. Geoscience Australia Record 2012/63. Canberra, Australia: Geoscience Australia; 2012. A reconnaissance crustal heat production assessment of the Australian Antarctic Territory (AAT) [Google Scholar]
- 72.Hodgson DA, et al. Were the Larsemann Hills ice-free through the Last Glacial Maximum? Antarct Sci. 2001;13(4):440–454. [Google Scholar]
- 73.Cromer L, Gibson JAE, Swadling KM, Hodgson DA. Evidence for a lacustrine faunal refuge in the Larsemann Hills, East Antarctica, during the Last Glacial Maximum. J Biogeogr. 2006;33(7):1314–1323. [Google Scholar]
- 74.Magurran AE. Measuring Biological Diversity. Oxford: Blackwell; 2004. [Google Scholar]
- 75.Rosenzweig ML. Species Diversity in Space and Time. Cambridge, UK: Cambridge Univ Press; 1995. [Google Scholar]
- 76.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85(7):1771–1789. [Google Scholar]
- 77.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 78.Bates D, Maechler M, Bolker B, Walker S. 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-4. Available at http://CRAN.R-project.org/package=lme4. Accessed January 14, 2014.
- 79.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. 2013. Generalized Linear Mixed Models using AD Model Builder. R package version 0.7.7. Available at http://glmmadmb.r-forge.r-project.org/. Accessed January 26, 2014.
- 80.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R Package. J Stat Softw. 2010;33(2):1–22. [Google Scholar]
- 81.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall; 2006. [Google Scholar]
- 82.Bivand RS, Pebesma EJ, Gómez-Rubio V. Applied Spatial Data Analysis with R. 2nd Ed. New York: Springer; 2013. [Google Scholar]
- 83.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 84.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64(4):583–616. [Google Scholar]
- 85.Ulrich W, Gotelli NJ. A null model algorithm for presence-absence matrices based on proportional resampling. Ecol Modell. 2012;244(0):20–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.