Abstract
Recent research suggests that cervical screening of older women is associated with a considerable decrease in cervical cancer incidence. We sought to quantify the efficacy of cervical cytology screening to reduce death from this disease. Among enrollees of 2 US health plans, we compared Papanicolaou smear screening histories of women aged 55–79 years who died of cervical cancer during 1980–2010 (cases) to those of women at risk of cervical cancer (controls). Controls were matched 2:1 to cases on health plan, age, and enrollment duration. Cytology screening during the detectable preclinical phase, estimated as the 5–7 years before diagnosis during which cervical neoplasia is asymptomatic but cytologically detectable, was ascertained from medical records. A total of 39 cases and 80 controls were eligible. The odds ratio of cervical cancer death associated with screening during the presumed detectable preclinical phase was 0.26 (95% confidence interval: 0.10, 0.63) after adjustment for matching characteristics, smoking, marital status, and race/ethnicity using logistic regression. We estimate that cervical cytology screening of all women aged 55–79 years in the United States could avert 630 deaths annually. These results provide a minimum estimate of the efficacy of human papillomavirus DNA screening—a more sensitive test—to reduce cervical cancer death among older women.
Keywords: case-control studies, Papanicolaou smear, screening, uterine cervical neoplasms, women's health
Cervical cancer screening by means of cytology, or the Papanicolaou (Pap) smear, seeks to detect precancerous or cancerous cervical lesions prior to symptom onset. Research has consistently observed that cervical cytology screening is highly efficacious against invasive cervical cancer (ICC) incidence and death among women of reproductive age (1–3). Data regarding the utility of screening older women for cervical cancer are limited. Analyses from Sweden and Finland suggest that participation in national organized cervical cancer screening programs is associated with 51%–64% reductions in cervical cancer incidence among older women (4, 5). In the United States, data from Kamineni et al. (6) suggest that cervical cancer screening among women aged 55–79 years is associated with a 77%–79% reduction in cervical cancer incidence.
Whether cytological screening reduces ICC death to a similar degree as its apparent reduction of ICC incidence has not been well evaluated in older women. Screening may preferentially detect slow-growing lesions and/or those lesions that are treatment responsive, in which case screening would not be as efficacious in reducing ICC death as its apparent reduction of incidence (7). However, 2 recent analyses suggest that cytology screening is associated with a substantial mortality benefit for older women. A case-only analysis of Sweden's cervical cancer screening registry found that women aged 66 years or older at diagnosis experienced a 36% increase in long-term survival if their cancers were detected by screening rather than clinically (8). A Finnish case-control study observed that the receipt of a negative Pap smear in the national screening program was associated with reduced cervical cancer death among women aged 55–69 years (9). Nonetheless, limitations in the information available from these national registries—such as lack of data on the presence of signs and/or symptoms at the time of each Pap smear, hysterectomy status, potential confounders, and the receipt of “opportunistic” smears outside national screening programs—restrict the conclusions that can be drawn from these results.
We sought to quantify the efficacy of cervical cancer screening to reduce cervical cancer death among older American women.
METHODS
Study design
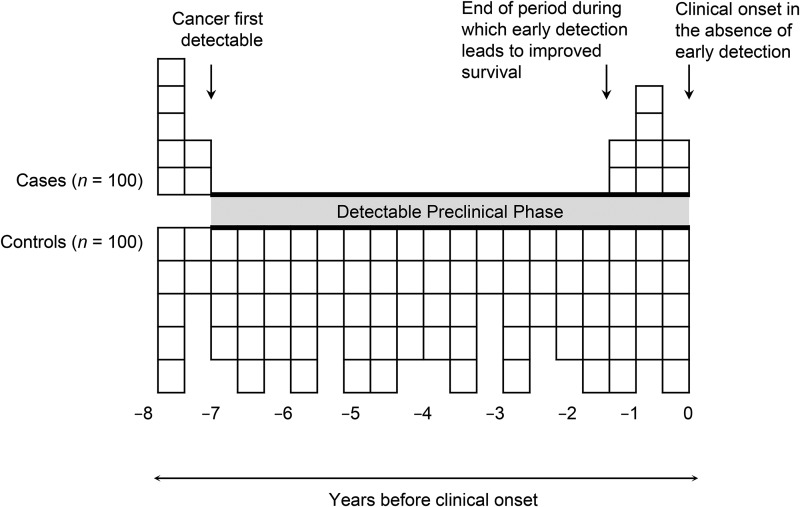
In this population-based case-control study, we compared cervical cytology screening histories of women who died of cervical cancer (cases) to those of a sample of women at risk of cervical cancer who were otherwise similar (controls) from 2 health plans in the US Pacific Northwest. Receipt of cervical cancer screening during the presumed detectable preclinical phase (DPP) of cervical cancer development was the primary exposure (bolded along the x-axis in Figure 1). For cervical cytology screening, the DPP begins when a premalignant lesion is detectable by cytology and ends with the onset of clinical signs or symptoms of ICC. A deficit of screening among cases relative to controls should be observable during this period if the test is beneficial (10, 11). Inclusion in the analysis of periods prior to or after the DPP attenuates the estimated benefit (10, 12).
Figure 1.
Screening during the detectable preclinical phase (DPP) of persons who died of their cancer (cases) and during the corresponding period among controls. The DPP is shown in bold along the x-axis. This figure assumes that the DPP is 7 years for all cases, that screening during the first 6 years of the DPP inevitably leads to the cancer being cured (i.e., odds ratio = 0), and that screening after that time is of no benefit to survival. Adapted from Weiss et al. (10).
Operationally, we first ascertained for each case the date of onset of clinical signs or symptoms that led to the diagnosis of cervical cancer (the index date) by means of a standardized medical record review. Cervical cancer signs or symptoms were defined as postmenopausal bleeding, postcoital bleeding, vaginal bleeding, nonspecific bleeding, abdominal pain, vaginal discharge, weight loss, obstructive uropathy, or ascites in the 12 months prior to cervical cancer diagnosis. If a case was screen detected, the screening date was the index date. The index date for each control matched that of her respective case. Second, whether a subject was screened for cervical cancer in the 7 years prior to the index date was ascertained from the medical records. Only screening tests (i.e., those performed in the absence of cervical cancer signs or symptoms) that occurred within 7 years before the index date were included in the analysis, because ICC incidence among Pap smear–negative women aged 55–79 years returns to that of unscreened women 5–7 years after a negative test. Numerous studies (6, 13, 14) have corroborated this 7-year estimate of the DPP for cervical cancer, though others have estimated that cervical cancer is detectable for up to 30 years (15). If the true DPP began more than 7 years before the index date—that is, if cervical cancer precursors were detectable by Pap smear for more than 7 years prior to the onset of cervical cancer signs or symptoms—then undercounting of screening Pap smears would be relatively greater among controls; controls’ screening is expected to have been relatively uniform during the case's DPP, whereas screening tests among cases would likely have occurred just prior to diagnosis, at the end of the DPP (Figure 1). Thus, if the presumed DPP was shorter than the true DPP, any difference in screening prevalence between cases and controls would be minimized, and the calculated odds ratio would underestimate the benefit of screening (10, 12).
Study population
Study subjects were identified from enrollees in 1 of 2 integrated health plans: Group Health, based in Seattle, Washington, and Kaiser Permanente Northwest (KPNW), based in Portland, Oregon, which together cover nearly 1.1 million individuals at present. Though these health plans’ screening algorithms recently incorporated human papillomavirus (HPV) DNA testing to triage equivocal cytology results, the result of an HPV DNA assay did not influence whether cytology was performed during any portion of the study period. Because Pap smears were administered independently of HPV DNA testing, the use of HPV DNA testing does not undermine this study's ability to ascertain the benefit of cytology screening.
Cases were women who died of cervical cancer during 1980–2010 at ages 55–79 years. Cases were ascertained from the Cancer Surveillance System for Group Health enrollees (part of the National Cancer Institute's Surveillance, Epidemiology, and End Results program) and from the Kaiser Tumor Registry for KPNW enrollees.
Controls were a sample of women aged 55–79 years enrolled in Group Health or KPNW during 1980–2010. To be a match, a control must have been enrolled in the health plan on her case's tumor registry diagnosis date (reference date). Matching was based on health plan, age (within 6 months), and duration of health plan enrollment prior to reference date (equal to or greater than that of the case by no more than 6 months). For both cases and controls, eligibility was restricted to women with 6 or more years of enrollment prior to the reference date, with no gaps in enrollment greater than 6 months. For Group Health controls, eligibility was restricted to women residing in the 13 western Washington counties surveyed by the Cancer Surveillance System. Eligible controls were ordered in terms of how well they were matched to the case, measured as the sum of the number of days between the case's and potential control's 1) birth dates and 2) health plan enrollment dates. The 2 potential controls with the lowest sums of these 2 numbers were selected for medical record review. Upon review, any potential control who had a hysterectomy prior to the reference date or who had evidence of receiving any health care outside the health plan was excluded and replaced with the next best-matched control, until 2 eligible controls with intact cervices were identified per case.
Cervical cancer screening history was recorded from medical records using a standardized medical record abstraction database developed with Microsoft Access, version 11.5, software (Microsoft Corp., Redmond, Washington). The reasons for the test, whether diagnostic or screening in nature, were ascertained for each Pap smear; only screening tests were included in the analysis (i.e., those performed in the absence of ICC signs or symptoms). Data on potential covariates were obtained from the medical record, including marital status, body mass index (weight (kg)/height (m)2), smoking history, race/ethnicity, parity, menopausal status, oral contraceptive use, and immunosuppressive status. Data were not available on sexual history or HPV infection. The institutional review boards at Group Health and KPNW each approved the study protocol.
Statistical analysis
Multivariate logistic regression was used to quantify the odds ratio of cervical cancer death associated with screening during the DPP, adjusting for matching variables and covariates that were associated (P < 0.10) with case status. Unconditional logistic regression was used because matching variables were easily quantified and conditional logistic regression would have decreased study efficiency (16, 17). Because the observed effect of screening may differ with estimated DPP length (10), sensitivity analyses were performed by varying the DPP duration in 6-month intervals from 5 to 7 years prior to the index date. Three exploratory analyses were planned: 1) stratification by age at diagnosis into less than 65 years or 65 years or older, the age at which the US Preventive Services Task Force (Rockville, Maryland), the American Cancer Society (Atlanta, Georgia), and the American Congress of Obstetricians and Gynecologists (Washington, DC) recommend cessation of screening for most women (18–20), 2) stratification by year of diagnosis (1999 or earlier vs. 2000 or later), because a major advance in cervical cancer treatment—the addition of chemotherapy to radiation therapy–based treatment—occurred in 1999 (21), and 3) restriction to cases with squamous cell carcinoma (SCC), because cytology screening may be more efficacious in preventing SCC than adenocarcinoma (22).
All statistical tests were 2-sided. The primary data analysis software used was Stata, version 12.0 (StataCorp LP, College Station, Texas).
RESULTS
Among 47 potentially eligible cases, 4 were excluded because no medical records were available, and 3 were excluded because they had likely received care outside the health plan. Among controls with sufficient enrollment duration, the most common reason for exclusion was evidence of hysterectomy before the reference date (n = 69), followed by lack of availability of medical records (n = 18), and documentation of outside health care (n = 16). These exclusions left 40 potential cases, and 80 controls were identified as eligible for inclusion in the study. After medical record abstraction, 1 case with less than 6 years of prediagnosis enrollment was excluded, leaving 39 cases and 80 controls available for the analysis.
There were no appreciable differences between cases and controls in most measured demographic characteristics (Table 1). A majority of study subjects (61%) were younger than 65 years at diagnosis; half of cases (49%) were younger than 65 years at death. Cases were more likely to be current smokers at the time of diagnosis (31%) than were controls (14%), and cases had higher body mass index values than controls (32% vs. 15% with body mass index ≥35). The overwhelming majorities of both cases (95%) and controls (97%) were white. Controls were more likely than cases to have been married at the reference date (68% vs. 56%). Cases were more likely than controls have had 3 or more births (72% vs. 50%). Among cases, the most common cervical cancer histology was SCC (51%), followed by adenocarcinoma (31%), undifferentiated carcinoma (10%), unknown histology (5%), and adenosquamous (3%) carcinoma.
Table 1.
Demographic Characteristics of Cervical Cancer Cases and Matched Controls, US Pacific Northwest, 1980–2010
| Characteristic | Cases (n = 39) |
Controls (n = 80) |
||
|---|---|---|---|---|
| No. | %a | No. | %a | |
| Health plan enrollment | ||||
| Group Healthb | 16 | 41.0 | 34 | 42.5 |
| Kaiser Permanente Northwestc | 23 | 59.0 | 46 | 57.5 |
| Age at diagnosisd, years | ||||
| 55–59 | 15 | 38.5 | 28 | 35.0 |
| 60–64 | 9 | 23.1 | 20 | 25.0 |
| 65–69 | 5 | 12.8 | 10 | 12.5 |
| 70–74 | 6 | 15.4 | 12 | 15.0 |
| ≥75 | 4 | 10.3 | 10 | 12.5 |
| Age at death, years | ||||
| 55–59 | 8 | 20.5 | NA | |
| 60–64 | 11 | 28.2 | NA | |
| 65–69 | 7 | 17.9 | NA | |
| 70–74 | 6 | 15.4 | NA | |
| ≥75 | 7 | 17.9 | NA | |
| Enrollment length prior to diagnosis, years | ||||
| <10 | 15 | 38.5 | 31 | 38.8 |
| 10–14 | 13 | 33.3 | 27 | 33.8 |
| ≥15 | 11 | 28.2 | 22 | 27.5 |
| Smoking history | ||||
| Never | 12 | 30.8 | 33 | 41.3 |
| Nonsmokere | 7 | 17.9 | 14 | 17.5 |
| Former smokerf | 8 | 20.5 | 22 | 27.5 |
| Current smoker | 12 | 30.8 | 11 | 13.8 |
| Body mass indexg | ||||
| <18.5 | 1 | 3.2 | 1 | 1.3 |
| 18.5–24.9 | 11 | 35.5 | 29 | 38.7 |
| 25.0–29.9 | 5 | 16.1 | 24 | 32.0 |
| 30.0–34.9 | 4 | 12.9 | 10 | 13.3 |
| ≥35.0 | 10 | 32.3 | 11 | 14.7 |
| Unknown | 8 | 5 | ||
| Race | ||||
| White | 36 | 94.7 | 74 | 97.4 |
| Hispanic | 1 | 2.6 | 0 | 0 |
| Asian | 1 | 2.6 | 2 | 2.6 |
| Unknown | 1 | 4 | ||
| Marital status | ||||
| Never married | 0 | 0 | 2 | 2.5 |
| Married | 20 | 55.6 | 54 | 68.4 |
| Divorced | 5 | 13.9 | 12 | 15.2 |
| Widowed | 10 | 27.8 | 11 | 13.9 |
| Separated | 1 | 2.8 | 0 | 0 |
| Unknown | 3 | 1 | ||
| Parity | ||||
| 0 | 2 | 5.1 | 9 | 11.3 |
| 1 | 2 | 5.1 | 8 | 10.0 |
| 2 | 7 | 17.9 | 23 | 28.8 |
| 3 | 11 | 28.2 | 12 | 15.0 |
| 4 | 10 | 25.6 | 13 | 16.3 |
| ≥5 | 17 | 17.9 | 15 | 18.8 |
Abbreviation: NA, not applicable.
a Percentage excludes unknowns, if any.
b Group Health is based in Seattle, Washington.
c Kaiser Permanente Northwest is based in Portland, Oregon.
d “At diagnosis” refers to the diagnosis date of the case or of the control's matched case (i.e., the reference date).
e Documented as a current nonsmoker as of index date; past smoking habits unknown.
f Documented as a current nonsmoker as of index date; documentation of smoking in the past.
g Body mass index is calculated as weight (kg)/height (m)2.
Screening histories differed substantially between cases and controls. In the 7 years prior to the index date, 51% of cases and 81% of controls received cervical cytology screening. The univariate odds ratio associated with 1 or more screens was 0.24 (95% confidence interval (CI): 0.10, 0.56) (Table 2). After adjustment for covariates (smoking status, marital status, and race/ethnicity), screening was associated with a 74% reduction in cervical cancer death (odds ratio (OR) = 0.26, 95% CI: 0.10, 0.63). Inclusion of all measured covariates did not alter the magnitude of the association (OR = 0.26, 95% CI: 0.09, 0.77).
Table 2.
Odds Ratios of the Association Between Cervical Cancer Screening During the 7 Years Prior to Index Date and Cervical Cancer Death Among US Women Aged 55–79 Years Using Multivariate Logistic Regression, 1980–2010
| Screening Status |
Cases (n = 39) |
Controls (n = 80) |
Univariate Modela |
Adjusted Modelb |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | OR | 95% CI | |
| Unscreened | 19 | 49 | 15 | 19 | 1.00 | Referent | 1.00 | Referent |
| Screened | 20 | 51 | 65 | 81 | 0.24 | 0.10, 0.56 | 0.26 | 0.10, 0.63 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for matching variables only (controls matched to cases on health plan (Group Health (Seattle, Washington) or Kaiser Permanente Northwest (Portland, Oregon)), duration of enrollment prior to diagnosis (continuous, in months), and age (continuous)).
b Adjusted for matching variables, plus those that were significant in the full model (P < 0.10): smoking status (never smoker, nonsmoker, former smoker, current smoker), marital status (never married, married, divorced, widowed, separated, unknown), race/ethnicity (white, Hispanic, Asian, unknown).
Exclusion of 10 subjects with less than 7 years of enrollment prior to the reference date did not affect the magnitude of the calculated odds ratio (adjusted OR = 0.26, 95% CI: 0.10, 0.67). In sensitivity analyses, the length of the DPP was varied from 5 to 6.5 years prior to the index date (Table 3). As the DPP shortened, the magnitude of the risk estimate was not appreciably affected. Similarly, the use of conditional logistic regression did not alter the primary odds ratio (Table 2) substantively (adjusted OR = 0.25, 95% CI: 0.09, 0.68), nor did it substantively alter the interpretation of any other analyses.
Table 3.
Sensitivity Analyses of Odds Ratios of the Association Between Cervical Cancer Screening During the 5–6.5 Years Prior to Index Date and Cervical Cancer Death Among US Women Aged 55–79 Years Using Multivariate Logistic Regression, 1980–2010a
| No. of Years Prior to Index Date That DPP Starts by Screening Status | Cases (n = 39) |
Controls (n = 80) |
Adjusted Modelb |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | |
| 6.5 | ||||||
| Unscreened | 20 | 51 | 18 | 22 | 1.00 | Referent |
| Screened | 19 | 49 | 62 | 78 | 0.30 | 0.12, 0.72 |
| 6 | ||||||
| Unscreened | 23 | 59 | 19 | 24 | 1.00 | Referent |
| Screened | 16 | 41 | 61 | 76 | 0.24 | 0.10, 0.57 |
| 5.5 | ||||||
| Unscreened | 25 | 64 | 20 | 25 | 1.00 | Referent |
| Screened | 14 | 36 | 60 | 75 | 0.20 | 0.08, 0.50 |
| 5 | ||||||
| Unscreened | 25 | 64 | 21 | 26 | 1.00 | Referent |
| Screened | 14 | 36 | 59 | 74 | 0.22 | 0.09, 0.53 |
Abbreviations: CI, confidence interval; DPP, detectable preclinical phase; OR, odds ratio.
a The interval during which screening was considered was varied in 6-month intervals, from 6.5 years prior to index date to 5 years prior to index date.
b Adjusted for matching variables, plus those that were significant in the full model (P < 0.10): smoking status (never smoker, nonsmoker, former smoker, current smoker), marital status (never married, married, divorced, widowed, separated, unknown), race/ethnicity (white, Hispanic, Asian, unknown).
In the first exploratory analysis, associations did differ somewhat by age (<65 years vs. ≥65 years at reference date; Table 4), but a reduced risk associated with screening was present in both age groups. Second, when subjects were stratified by year of diagnosis (1999 or earlier vs. 2000 or later), the reduction in cervical cancer death associated with screening was similar during the 2 intervals (Table 4). Finally, restriction to cases with SCC tumor histology (n = 20) yielded a lower adjusted odds ratio (OR = 0.13, 95% CI: 0.03, 0.54) (Table 5).
Table 4.
Odds Ratios of the Association Between Cervical Cancer Screening During the 7 Years Prior to Index Date and Cervical Cancer Death, Stratified by Age at Diagnosis and Year of Diagnosis, US Pacific Northwest, 1980–2010
| Variable | Cases (n = 39) |
Controls (n = 80) |
Adjusted Modela |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | |
| Age at diagnosis, years | ||||||
| <65 | ||||||
| Unscreened | 14 | 58 | 8 | 17 | 1.00 | Referent |
| Screened | 10 | 42 | 40 | 83 | 0.18 | 0.06, 0.57 |
| ≥65 | ||||||
| Unscreened | 5 | 33 | 7 | 23 | 1.00 | Referent |
| Screened | 10 | 67 | 25 | 78 | 0.47 | 0.14, 1.63 |
| Year of diagnosis | ||||||
| 1999 or earlier | ||||||
| Unscreened | 12 | 57 | 8 | 19 | 1.00 | Referent |
| Screened | 9 | 43 | 34 | 81 | 0.23 | 0.08, 0.67 |
| 2000 or later | ||||||
| Unscreened | 7 | 39 | 7 | 18 | 1.00 | Referent |
| Screened | 11 | 61 | 31 | 82 | 0.29 | 0.10, 0.84 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for matching variables, plus those that were significant in the full model (P < 0.10): smoking status (never smoker, nonsmoker, former smoker, current smoker), marital status (never married, married, divorced, widowed, separated, unknown), race/ethnicity (white, Hispanic, Asian, unknown).
Table 5.
Odds Ratios of the Association Between Cervical Cancer Screening During the 7 Years Prior to Index Date and Cervical Cancer Death, Restricted to Cases With Squamous Cell Carcinoma Pathology, US Pacific Northwest, 1980–2010
| Screening Status |
Cases |
Controls |
Adjusted Modela |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | |
| Unscreened | 11 | 55 | 6 | 15 | 1.00 | Referent |
| Screened | 9 | 45 | 34 | 85 | 0.13 | 0.03, 0.54 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted for matching variables, plus those that were significant in the full model (P < 0.10): smoking status (never smoker, nonsmoker, former smoker, current smoker), marital status (never married, married, divorced, widowed, separated, unknown), race/ethnicity (white, Hispanic, Asian, unknown).
DISCUSSION
In this study, we observed that cervical cancer screening by cytology during the DPP, estimated as the 7 years prior to symptom onset or screen-detected diagnosis, was associated with a 74% reduction in cervical cancer death among women aged 55–79 years at death. This reduction is nearly identical to the estimate by Kamineni et al. (6) of efficacy with respect to incidence (77%) from the same 2 health plans and during a similar time period (1980–1999). The similarity between the 2 estimates suggests that cervical cytology screening in older women does not preferentially detect slow-growing and/or treatment-responsive lesions.
This study was designed to answer a direct and interpretable question of public health significance. Outcome and exposure data were drawn from valid sources: established tumor registries and medical record review, respectively. Previous research has repeatedly documented that the medical record is a more accurate source of Pap smear screening history than self-report (23–26). Unlike in prior studies addressing this research question (8, 9), in this study we ascertained hysterectomy status and the presence of cervical cancer signs and/or symptoms at the time of each cytology test, and women who had any evidence of care outside the health plan were excluded in an attempt to ensure that complete exposure information could be ascertained. The results were robust; when subjects with less than 7 years of enrollment prior to the reference date were excluded, or the length of the DPP was shortened, results did not change meaningfully. Further, the observed 74% decrease in cervical cancer death associated with screening may actually be an underestimate if the DPP duration is truly longer than 7 years (10, 12).
Given the small number of total cases in the main analysis, the precision of the 3 prespecified exploratory analyses was limited. First, although the efficacy among women aged 65 years or older was less than that among women under 65 years of age, the confidence intervals of these estimates preclude any strong inferences about the differential efficacy of cytology screening by age. Indeed, other cervical screening studies have observed that screening efficacy is not attenuated and may actually increase with age among older women (3–6), though the differences in risk estimates by age were not of large magnitude (nor statistically significant), and these studies examined the outcome of incidence rather than death. The 2 studies that examined cervical cancer death found comparable trends by age; Andrae et al. (8) observed a higher cure proportion associated with screen detection among women aged 66 years or older compared with those aged 23–65 years (36%, 95% CI: 11, 80 vs. 26%, 95% CI: 15, 36, respectively), and the results of Lönnberg et al. (9) were consistent with similar efficacy among women aged 55–69 years compared with women aged 40–54 years (OR = 0.29, 95% CI: 0.16, 0.54 vs. OR = 0.33, 95% CI: 0.20, 0.56). Second, the similarity of the odds ratios in the 2 time intervals, 1999 or earlier versus 2000 or later, supports the inference that improvements in cervical cancer treatment—primarily, the addition of chemotherapy to radiation-based treatment regimens—have not rendered screening relatively less efficacious with respect to death. Third, the somewhat higher efficacy observed for SCC cases corroborates other studies’ conclusions (22, 27) that cytology screening is likely more efficacious at detecting SCC than adenocarcinoma.
This study did have limitations. No data were available on sexual history or HPV, the etiological agent of cervical cancer (28). However, neither of these variables is routinely ascertained for postmenopausal women; therefore, neither is likely to influence a clinician's decision to screen for cervical cancer. During the study period, clinical screening guidelines at Group Health and KPNW did not include ascertaining high-risk HPV status. These guidelines did consider age at sexual debut, but this influenced only age at screening initiation, not cessation. For these reasons, lack of ascertainment of sexual history and HPV status are not likely to confound the measured odds ratios. Smoking is the most plausible confounder, because it is a strong ICC risk factor (29). Smoking status was ascertained for all subjects. Second, the 7-year estimate of the DPP of cervical cancer screening by means of cytology may underestimate the true DPP. However, data from the United Kingdom (14), the United States (6) and a worldwide analysis (13) indicate that 1 or 2 negative Pap smears predicts low risk of disease for no more than 7 years, and sensitivity analyses in this study did not indicate that shortening the DPP duration affects the odds ratio substantially (Table 3). If cervical cancer or its precursors were detectable via Pap smear for more than 7 years prior to the index date, a greater proportion of screening Pap smears would be missed among controls than among cases (Figure 1), minimizing any difference between the comparison groups and thus attenuating the odds ratio (12). Finally, this study could not address the impact of recent screening as a function of the adequacy of screening earlier in life, because data on screening prior to health plan enrollment were not available. However, previous research indicates that older women experience a low incidence of cervical cancer for no more than 7 years following 1 or 2 negative Pap smears (6, 13, 14). Further, the cure proportion did not differ with prior cytology screening history among women aged 66 years or older at diagnosis (8). Thus, it is reasonable to infer that the results of the present study are applicable to older women regardless of prior Pap smear screening history.
Irrespective of the study's strengths and the robustness of its results, some may consider its research question obsolete. Cytological screening will most likely decline in favor of HPV-based screening because of its superiority over cytology in the 2 characteristics that influence test efficacy (7); HPV DNA testing can detect ICC risk for a longer period than cytology (30, 31), and its sensitivity is an absolute 40% higher than that of cytology (32, 33). Thus, the relationship between these screening modalities’ efficacies is knowable—the efficacy of HPV-based screening is expected to exceed that of cytology, all things being equal. Analysis of extant data on cytology screening, therefore, may offer a minimum estimate of HPV-based screening efficacy among older women. However, screening by cytology alone remains acceptable under all current guidelines, and Pap smears continue to be widely used (18, 19, 34). Further, a study to evaluate the efficacy of HPV DNA testing among older women will not be possible for years after an HPV DNA–based screening program is implemented, until a sufficient number of deaths have occurred to make meaningful comparisons on the basis of prior HPV DNA screening history. Until such data are available, this study's results may shed light on the potential efficacy of an HPV-based screening program to prevent cervical cancer death among older women. In effect, the present study provides insight into whether to screen older women, not the best modality to do so.
In the meantime, cancer screening guideline groups acknowledge that lack of evidence hampers their ability to make evidence-based recommendations for cervical cancer screening in older women. The present study addresses this research gap. On the basis of this study's odds ratios and the screening prevalence among the cases, and assuming the observed association is causal, 36.2% of cervical cancer deaths among women aged 55–79 years in the United States, or approximately 630 deaths per year (35), could have been averted by screening in the 7 years prior to symptom onset or screen-detected diagnosis. It is likely that a larger number of deaths could be averted with an HPV-based screening strategy.
The results of this study, in conjunction with those from previous analyses, suggest a potential benefit of extending screening guidelines to include women older than 65 years. Cervical cytology screening is not without harms, such as invasive diagnostic procedures, short-term psychological distress, and overdiagnosis (19). If, after quantification of the potential harms of screening older women, they are deemed to be outweighed by the potential benefits, national guideline groups could consider an expansion of the age group for which cervical cancer screening is currently recommended.
ACKNOWLEDGMENTS
Author affiliations: Department of Global Health, Schools of Medicine and Public Health, University of Washington, Seattle, Washington (Alison S. Rustagi); Group Health Research Institute, Group Health, Seattle, Washington (Aruna Kamineni); Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon (Sheila Weinmann); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Susan D. Reed, Polly Newcomb, Noel S. Weiss); Department of Obstetrics and Gynecology, School of Medicine, University of Washington, Seattle, Washington (Susan D. Reed); Cancer Prevention Program, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Polly Newcomb); and Cancer Epidemiology Research Cooperative, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Susan D. Reed, Noel S. Weiss).
This work was supported by the National Cancer Institute (grants R25 CA094880, K05 CA092002); and the National Center for Advancing Translational Sciences (grant TL1 TR 000422) at the National Institutes of Health.
We thank Kristin Delaney at Group Health and Erin Masterson at Kaiser Permanente Northwest for their programming expertise and time, as well as Beth Kirlin at Group Health and Denise Schwarzkopf at Kaiser Permanente Northwest for their work as project managers.
This research was presented at the International Cancer Screening Network biennial meeting in Sydney, Australia, October 23–25, 2012.
Conflict of interest: none declared.
REFERENCES
- 1.Day NE. Screening for cancer of the cervix. J Epidemiol Community Health. 1989;43(2):103–106. doi: 10.1136/jech.43.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafsson L, Pontén J, Zack M, et al. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8(5):755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 3.Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ. 2009;339:b2968. doi: 10.1136/bmj.b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrae B, Kemetli L, Sparén P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100(9):622–629. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 5.Lönnberg S, Anttila A, Luostarinen T, et al. Age-specific effectiveness of the Finnish cervical cancer screening programme. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1354–1361. doi: 10.1158/1055-9965.EPI-12-0162. [DOI] [PubMed] [Google Scholar]
- 6.Kamineni A, Weinmann S, Shy KK, et al. Efficacy of screening in preventing cervical cancer among older women. Cancer Causes Control. 2013;24(9):1653–1660. doi: 10.1007/s10552-013-0239-4. [DOI] [PubMed] [Google Scholar]
- 7.Morrison AS. Screening in Chronic Disease. 2nd ed. New York, NY: Oxford University Press; 1992. [Google Scholar]
- 8.Andrae B, Andersson TM, Lambert PC, et al. Screening and cervical cancer cure: population based cohort study. BMJ. 2012;344:e900. doi: 10.1136/bmj.e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lönnberg S, Nieminen P, Luostarinen T, et al. Mortality audit of the Finnish cervical cancer screening program. Int J Cancer. 2013;132(9):2134–2140. doi: 10.1002/ijc.27844. [DOI] [PubMed] [Google Scholar]
- 10.Weiss NS, McKnight B, Stevens NG. Approaches to the analysis of case-control studies of the efficacy of screening for cancer. Am J Epidemiol. 1992;135(7):817–823. doi: 10.1093/oxfordjournals.aje.a116368. [DOI] [PubMed] [Google Scholar]
- 11.Weiss NS, Lazovich D. Case-control studies of screening efficacy: the use of persons newly diagnosed with cancer who later sustain an unfavorable outcome. Am J Epidemiol. 1996;143(4):319–322. [PubMed] [Google Scholar]
- 12.Etzioni RD, Weiss NS. Analysis of case-control studies of screening: impact of misspecifying the duration of detectable preclinical pathologic changes. Am J Epidemiol. 1998;148(3):292–297. doi: 10.1093/oxfordjournals.aje.a009638. [DOI] [PubMed] [Google Scholar]
- 13.IARC Working Group on Cervical Cancer Screening. Screening for squamous cervical cancer—the duration of low risk following negative results in cervical cytology test: introduction. IARC Sci Publ. 1986;(76):15–24. [PubMed] [Google Scholar]
- 14.Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer. 2003;89(1):88–93. doi: 10.1038/sj.bjc.6600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 16.Prentice R. Use of the logistic model in retrospective studies. Biometrics. 1976;32(3):599–606. [PubMed] [Google Scholar]
- 17.Lynn HS, McCulloch CE. When does it pay to break the matches for analysis of a matched-pairs design? Biometrics. 1992;48(2):397–409. [PubMed] [Google Scholar]
- 18.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer VA U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 20.Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 131: screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists. ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas. Number 35, May 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;78(1):79–91. doi: 10.1016/s0020-7292(02)90092-5. [DOI] [PubMed] [Google Scholar]
- 22.Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009;125(3):525–529. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 23.Walter SD, Clarke EA, Hatcher J, et al. A comparison of physician and patient reports of Pap smear histories. J Clin Epidemiol. 1988;41(4):401–410. doi: 10.1016/0895-4356(88)90148-5. [DOI] [PubMed] [Google Scholar]
- 24.McKenna MT, Speers M, Mallin K, et al. Agreement between patient self-reports and medical records for Pap smear histories. Am J Prev Med. 1992;8(5):287–291. [PubMed] [Google Scholar]
- 25.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85(7):566–570. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]
- 26.Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 27.International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120(4):885–891. doi: 10.1002/ijc.22357. [DOI] [PubMed] [Google Scholar]
- 28.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 90. Human Papillomaviruses. Lyon, France: IARC Press; 2007. [Google Scholar]
- 29.Appleby P, Beral V, et al. International Collaboration of Epidemiological Studies of Cervical Cancer. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 30.Schiffman M, Wheeler CM, Castle PE, et al. Human papillomavirus DNA remains detectable longer than related cervical cytologic abnormalities. J Infect Dis. 2002;186(8):1169–1172. doi: 10.1086/343816. [DOI] [PubMed] [Google Scholar]
- 31.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 32.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 33.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 34.Saint M, Gildengorin G, Sawaya GF. Current cervical neoplasia screening practices of obstetrician/gynecologists in the US. Am J Obstet Gynecol. 2005;192(2):414–421. doi: 10.1016/j.ajog.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. Deaths: Final Data for 2010. Hyattsville, MD: National Center for Health Statistics; 2013. (National vital statistics reports, volume 61, issue 4) (DHHS publication no. (PHS) 2013-1120) [PubMed] [Google Scholar]