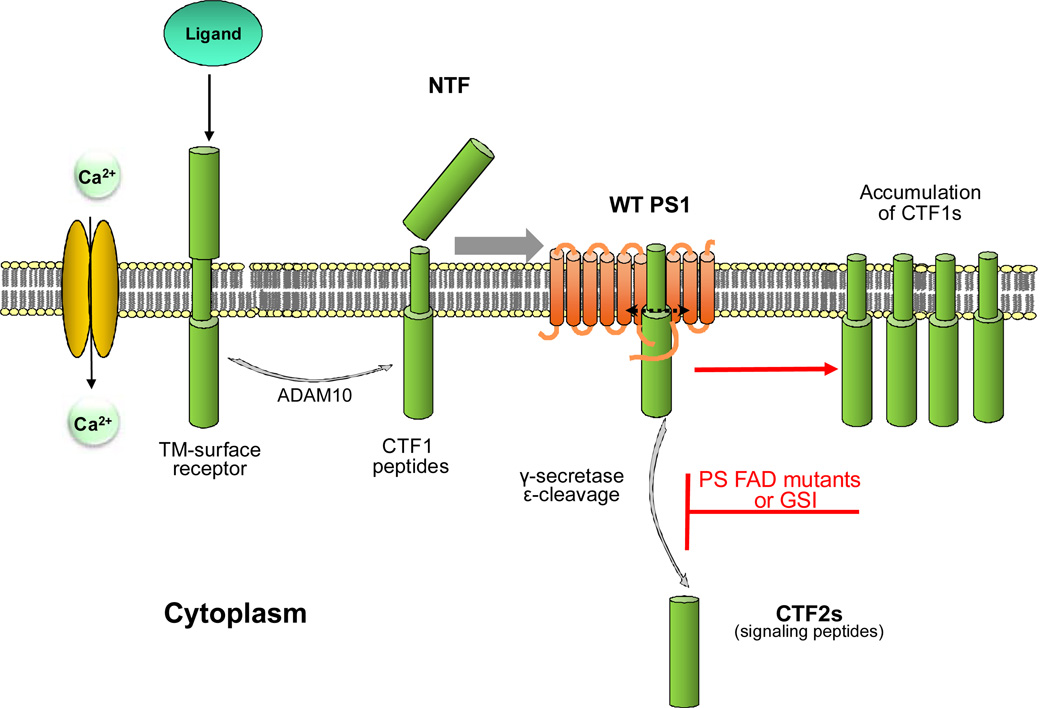
Figure 1.
Extracellular cleavage of cell surface proteins by MP ADAM10 produces TM CTF1 peptides that can be processed at ε sites by γ-secretase to produce cytosolic CTF2 peptides. The ADAM10 cleavage may be constitutive but also activated by ligand binding to receptors or calcium influx through NMDAR (2). Inhibition of the γ-secretase-dependent ε cleavage of CTF1s by either dominant heterozygous FAD mutations (see text) or GSIs may cause cytotoxicity by decreasing functional CTF2 peptides and promoting accumulation of unprocessed CTF1 substrates of γ-secretase. MP: metalloproteinase; TM: transmembrane; CTF: carboxy-terminal fragments of protein; NTF: N-terminal fragment of protein; GSI: γ secretase inhibitors; WT: wild type