Figure 4.
Ena and Dia Directly Bind and Interact in D16 Cells
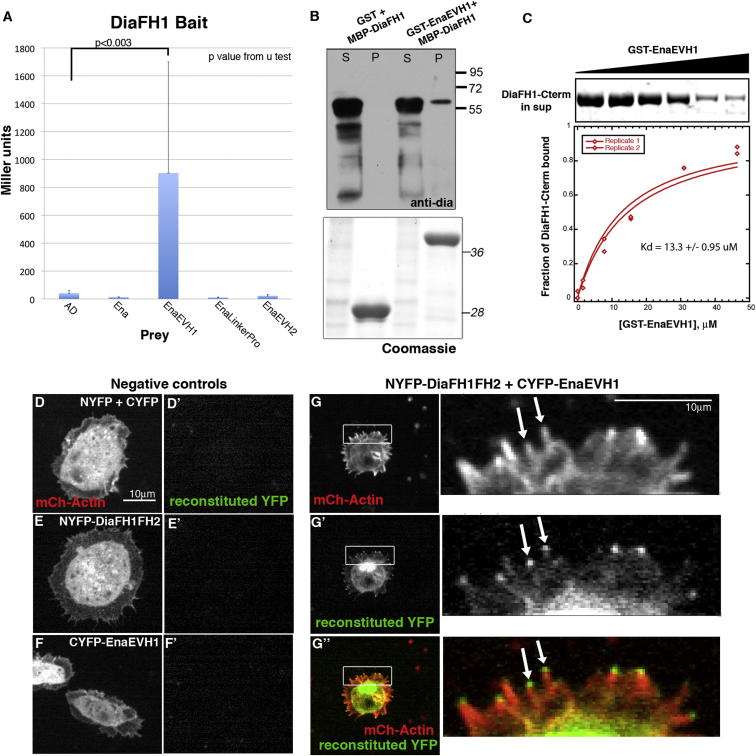
(A) Yeast two-hybrid βgal assays with DiaFH1 bait. Mean Miller units ± SD.
(B) Top: GST-EnaEVH1 pulls down MBP-DiaFH1; S, supernatant; p, pellet. Bottom: Coomassie verifying equal loading.
(C) Purified DiaFH1-Cterm is pulled down by GST-EnaEVH1. Top: Coomassie stained gel of DiaFH1-Cterm recruitment from supernatant with increasing concentrations of GST-EnaEVH1. Bottom: plot of dependence of DiaFH1-Cterm bound over a range of GST-EnaEVH1 concentrations. Average equilibrium dissociation constant = 13.3 μM.
(D–G″) Split YFP in D16 cells. mCh-Actin (D, E, F, G) and reconstituted YFP fluorescence (D′, E′, F′, and G′) in NYFP+CYFP (D and D′), NYFP-DiaFH1FH2 alone (E and E′), CYFP-EnaEVH1 alone (F and F′), and NYFP-DiaFH1FH2+CYFP-EnaEVH1 (G–G″). Arrows in inset, YFP at filopodia tips.
See Table S1.