Abstract
Motion cues provide a rich source of information about translations of the observer through the environment as well as the movements of objects and surfaces. While the direction of motion can be extracted locally these local measurements are, in general, insufficient for determining object and surface motions. To study the development of local and global motion processing mechanisms, we recorded Visual Evoked Potentials (VEPs) in response to dynamic random dot displays that alternated between coherent rotational motion and random motion at 0.8 Hz. We compared the spatio-temporal tuning of the evoked response in 4-6 month old infants to that of adults by recording over a range of dot displacements and temporal update rates. Responses recorded at the frequency of the coherent motion modulation were tuned for displacement at the occipital midline in both adults in infants. Responses at lateral electrodes were tuned for speed in adults, but not in infants. Infant responses were maximal at a larger range of spatial displacement than that of adults. In contrast, responses recorded at the dot update rate showed a more similar parametric displacement tuning and scalp topography in infants and adults. Taken together, our results suggest that while local motion processing is relatively mature at 4-6 months, global integration mechanisms exhibit significant immaturities at this age.
Keywords: Motion coherence, Infant vision, Visual development, Visual evoked potentials
Introduction
Motion sensitivity, like spatial vision, is a fundamental aspect of visual perception. A crude form of direction selectivity can be demonstrated in V1 cells of infant macaques at 1-2 wks of age (Chino, Smith, Hatta & Cheng, 1997), in very young visually inexperienced kittens (Hubel & Wiesel, 1963) and in visually naïve ferrets (Li, Van Hooser, Mazurek, White & Fitzpatrick, 2008). Visual experience with moving contours however is critical for refining and maintaining cortical direction selectivity (Cynader, Berman & Hein, 1973, Cynader & Chernenko, 1976, Humphrey & Saul, 1998, Humphrey, Saul & Feidler, 1998, Li, Fitzpatrick & White, 2006, Li et al., 2008), as is normal binocular interaction (Watanabe, Bi, Zhang, Sakai, Mori, Harwerth, Smith & Chino, 2005).
Assessment of direction selective mechanisms in humans is necessarily more indirect, and different assays suggest different developmental time courses. Directional optokinetic eye movement responses (OKN) can be elicited in newborns (Kremenitzer, Vaughan, Kurtzberg & Dowling, 1979, Naegele & Held, 1982, Phillips, Finocchio, Ong & Fuchs, 1997, Volkmann & Dobson, 1976). However, because OKN in primates is controlled by a combination of cortical and subcortical motion systems (Distler, Vital-Durand, Korte, Korbmacher & Hoffmann, 1999), it is not clear which system is responsible for neonatal OKN. Other directional eye movements can also be elicited near birth in humans (Rosander, 2007), but again the locus of control is uncertain. Using Visual Evoked Potentials (VEPs), (Wattam-Bell, 1991) found evidence for cortical direction selectivity in infants by the age of 10 weeks for a stimulus velocity of 5 deg/s and by the age of 12 weeks for a stimulus velocity of 20 deg/s, suggesting that the development of directionality proceeds from low to high velocities. A more recent VEP study has found that direction-reversal responses appeared in less than 25% of infants under 7 weeks of age, rising to 80% or more at 11-13 weeks (Braddick, Birtles, Wattam-Bell & Atkinson, 2005). The monocular oscillatory motion VEP displays a directional bias in older infants (Norcia, Garcia, Humphry, Holmes, Hamer & Orel-Bixler, 1991), but not before about one month of age (Birch, Fawcett & Stager, 2000), suggesting that cortical direction selectivity emerges post-natally in humans. Finally, a number of behavioral preference studies provide evidence for directional motion sensitivity within the first 3 months of life in humans (Braddick, Atkinson & Wattam-Bell, 2003).
A common strategy for studying motion selectivity in human is to use population response measures such as fMRI and visual evoked potentials/magnetic fields with experimental designs that contrast responses to coherent versus incoherent motion in dynamic random dot kinematograms (Aspell, Tanskanen & Hurlbert, 2005, Braddick, O'Brien, Wattam-Bell, Atkinson, Hartley & Turner, 2001, Braddick, O'Brien, Wattam-Bell, Atkinson & Turner, 2000, Handel, Lutzenberger, Thier & Haarmeier, 2007, Koyama, Sasaki, Andersen, Tootell, Matsuura & Watanabe, 2005, Lam, Kaneoke, Gunji, Yamasaki, Matsumoto, Naito & Kakigi, 2000, Morrone, Tosetti, Montanaro, Fiorentini, Cioni & Burr, 2000, Nakamura, Kashii, Nagamine, Matsui, Hashimoto, Honda & Shibasaki, 2003, Niedeggen & Wist, 1999). Coherent motion displays contain two types of motion signals, a local one involving short-range correlations in the apparent motion of individual dots in the pattern and a global one involving a systematic organization of the local motion vectors into flow fields (Newsome & Pare, 1988). Differential responses to coherent versus incoherent motion displays indicates successful encoding of both the local direction signals as well as their global organization.
Behavioral sensitivity to coherent motion has been demonstrated within the second month of life (Banton & Bertenthal, 1996, Banton, Bertenthal & Seaks, 1999, Wattam-Bell, 1994, Wattam-Bell, 1996). A longitudinal behavioral study in macaque infants aged between 10 days and 3 years found that coherent motion sensitivity continued to improve up to at least 3 years of age (Kiorpes & Movshon, 2004). The sensitivity of the youngest monkeys was highest at large dot displacements and fast speeds and coherence sensitivity improved for small dot displacements and slow speeds with age. In humans, development of psychophysical sensitivity is incomplete in middle childhood, especially at slow speeds (Atkinson, 2000, Ellemberg, Lewis, Dirks, Maurer, Ledgeway, Guillemot & Lepore, 2004, Ellemberg, Lewis, Maurer, Brar & Brent, 2002, Gunn, Cory, Atkinson, Braddick, Wattam-Bell, Guzzetta & Cioni, 2002).
Both single-unit recording studies (Duffy & Wurtz, 1995, Heuer & Britten, 2004, Snowden, Treue & Andersen, 1992, Tanaka & Saito, 1989) and human functional imaging studies (Braddick et al., 2000, Goossens, Dukelow, Menon, Vilis & van den Berg, 2006, Morrone et al., 2000, Seiffert, Somers, Dale & Tootell, 2003) indicate that sensitivity to global structure in coherent motion displays is greatest in extra-striate cortical areas. Given the hierarchical nature of the coherent motion stimulus, and the relative specificity of global responses in extra-striate areas, these two responses, one “global” and the other “local” likely reflect different visual processing mechanisms located at different levels in the visual pathway. In our experiment, both the spatial and temporal displacements of the local apparent motion cue were varied parametrically in such a way that we could determine the overall pattern of spatio-temporal dependence of both local responses -- those that were time-locked to the dot-update rate (15, 20 or 30 Hz) and global responses --- those that were time-locked to the global-update rate (0.8 Hz) at which the directional coherence modulated. More specifically, the stimulus parameters were chosen to provide a strong test of whether the local or global response tuning depended separately on spatial and temporal displacement or on speed. Speed sensitivity is likely to have relevance behaviorally but it is unclear at present whether the evoked response of either adults or infants shows evidence of explicit coding of speed or whether it reflects more basic parameters of spatial and temporal displacement. Moreover, changes in speed sensitivity could occur due to development in spatial or temporal resolution or both.
We find that the infant response to modulations of motion coherence is maximal at larger spatial displacements than that of the adults, consistent with Kiorpes and Movshon's behavioral study in the macaque. We also find that the adult response to coherence modulation is speed-tuned at lateral electrodes. Local motion sensitivity, on the other hand is adult-like in terms of its spatio-temporal tuning. Together our results suggest that the first stages of local motion extraction are relatively mature by 4-6 months, but that significant immaturities are present in the mechanisms, which we presume to lie primarily in extra-striate cortex, that encode the global organization of the local motion vectors.
Methods
Observers
A total of 36 healthy full-term infants between 17-24 weeks of age (mean age: 21 wks ± 2.3 wks) and 14 adults with normal or corrected to normal vision between 17-53 years of age (mean age: 34 yrs ± 11 yrs) participated. The research protocol was approved by the Institutional Review Board of the California Pacific Medical Center and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the parents of the infants and the adult observers after the VEP recording procedure was explained.
Stimuli and Apparatus
The participants viewed random-dot kinematograms displayed on a color CRT monitor running in monochrome mode (640 × 480 pixel resolution, 120 Hz refresh rate). The active display area was 24 deg in diameter at a 70 cm viewing distance for both adults and infants. The random-dot kinematograms were composed of 12.4 arc min white dots (105 cd/m2) on a black background (5 cd/m2). Dot density was 10% of the screen area (3 dots/deg). A small fixation mark was presented in the center of the display.
The display alternated between circular coherent motion and incoherent motion at 0.83 Hz, with the direction of coherent motion alternating, e.g. 0.6 s of clockwise motion followed by 0.6 s of random motion, followed by 0.6 s of counter-clockwise motion followed by 0.6 s or random motion, etc. in order to reduce the effects of motion adaptation (see Figure 1). A full stimulus cycle thus lasted 2.4 sec, but the data were averaged across the two directions of motion to yield a single 0.83 Hz cycle of coherent/incoherent alternation. Five stimulus cycles were shown one after the other in a trial lasting 12 sec. All dots of both the random and coherent motion displays were updated at 15, 20 or 30 Hz and remained stationary for periods equal to the reciprocal of the update rate. Each dot was displaced by a fixed distance in a given block of trials. In the coherent portions of the displays, all dots rotated either clockwise or anticlockwise at 100% coherence. In the random motion portions, the dot direction was randomized over 360 deg on each update (Brownian motion). A given dot persisted across both coherent and incoherent motion episodes, only its direction differed. Dot lifetime was 3 seconds but a small fraction of the dots was randomly re-plotted on every update. The continuity of dot lifetime across motion types, coupled with the random replacement rule ensured that there were no artifactual image transients specifically time-locked to the global motion update rate.
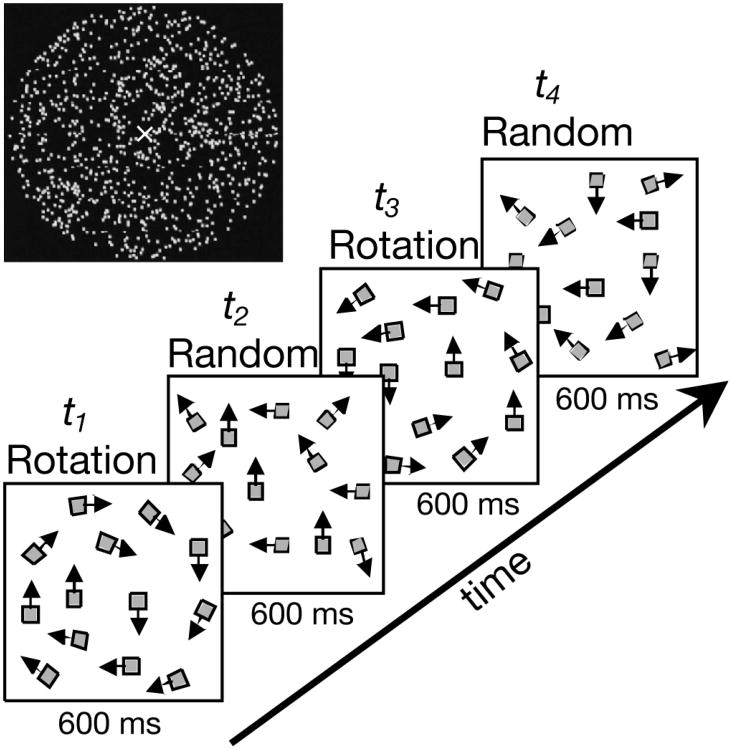
Figure 1.
Schematic illustration of the stimuli. A random-dot kinematogram was used. A full cycle of the stimulus consisted of 0.6 s of circular coherent motion (clockwise) followed by 0.6 s of incoherent motion, followed by 0.6 s of anticlockwise motion, followed by 0.6 s of incoherent motion. The position of individual dots was shifted over a fixed spatial displacement at each temporal update in both coherent and incoherent phases of the display. VEP responses were measured over a wide range of dot displacements (Δx) at three dot-update frequencies (1/Δt's of 15, 20 and 30 Hz).
VEP responses were measured over a wide range of spatial displacements (interdot spacings, Δx) of 3.1 arc min to 74.4 arc min at temporal displacements (dot update rates, 1/Δt) of 15, 20 and 30 Hz. The combination of Δx and Δt values resulted in a range of speeds from 0.8 deg/s to 18.6 deg/s. The conditions tested are indicated in diagrammatic form in Figure 2. We will refer to the coherent/incoherent motion stimulus alternation at 0.83 Hz as the global-update rate or FG and the apparent motion at the 15, 20 and 30 Hz rates as the dot-update or local-update rate or FL. These terms are used for convenience and are not meant to imply any particular processing mechanism (see Discussion). VEPs were recorded binocularly at 90% contrast (Michelson definition).
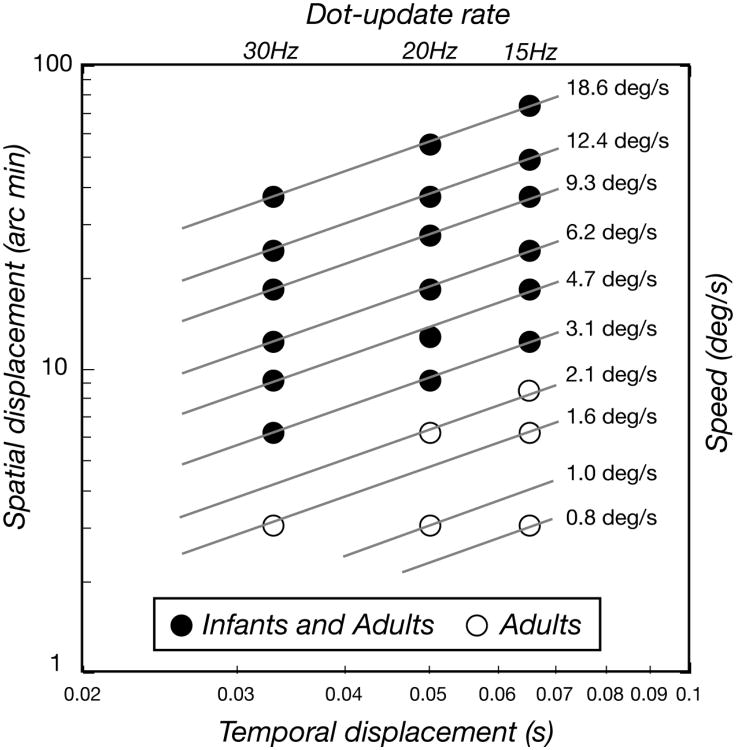
Figure 2.
Stimulus conditions. Local apparent motion occurred over a range of spatial displacements (Δx) and temporal displacements (Δt) arranged to systematically probe a number of Δx's at a fixed Δt (vertical columns), a number of Δt's at a fixedΔx (horizontal rows) and combinations of Δx and Δt that lead to a consistent speed (diagonal lines). Both infants and adults provided data for the conditions indicated by the filled circles. Adults provided additional data in the conditions indicated by the open circles.
VEP recording and procedure
VEPs were recorded at PO7, O1, Oz, O2 and PO8 with respect to a reference at Cz (International 10-10 electrode placement system (Nuwer, Comi, Emerson, Fuglsang-Frederiksen, Guerit, Hinrichs, Ikeda, Luccas & Rappelsburger, 1998). Impedance was measured and maintained between 3 and 10 Kohms. The EEG was amplified at a gain of 20,000 for infants and 50,000 for adults, with amplitude band-pass-filter settings of 0.3 to 100 Hz at -6dB (Model 12 A5; Grass Instruments, Quincy, MA). The EEG was digitized to a nominal 16-bit accuracy at a sampling rate of 500 Hz.
Infants were seated in their parent's lap in front of the monitor. The experimenter attracted the infant's attention to the stimulus with small toys centered on the display. Recordings were interrupted when the infant was judged not to be attending. If the experimenter interrupted the display with a mouse input, both display and data acquisition program loops were reset to a previous point in the display that was at least 1 s prior to the mouse press.
Due to limitations in the length of time that we could hold the infants in a state of quiet attentiveness, it was not possible to perform a detailed parametric study of a wide range of Δx and Δt using a within-observer design. We reduced the individual experiments to manageable proportions for use with infants by a combination of within- and between-observer designs and the selection of key stimulus contrasts. Spatial displacement tuning curves were recorded at the 15, 20 and 30 Hz update rates in three separate groups of 12 infants. These infants each participated in two recording sessions that occurred within 1-2 wks of each other during which we recorded 4-8 trials per condition. VEPs were recorded over three sessions in the adults. In each session, a tuning curve was measured at the 15, 20 or 30Hz dot-update rate. Ten trials were recorded per stimulus condition.. The trials were randomly interleaved across conditions in blocks of 2 trials for infants and 5 trials for the adults.
VEP Analysis
A recursive least squares (RLS) adaptive filter (Tang & Norcia, 1995) was used to determine VEP amplitude and phase for the first 5 harmonics (1FG, 2 FG, 3FG, 4FG and 5FG) of the 0.83 Hz global motion frequency and the first harmonic (1FL) of the 15, 20 and 30 Hz local motion frequencies. The RLS filter optimally adjusts the weights of the real and imaginary response components to best match the recorded data at each frequency of interest.
Mean VEP amplitudes for each observer for each separate electrode, harmonic, and stimulus condition were obtained by coherently averaging the real and imaginary spectral coefficients for each trial. The individual observer mean response vectors were pooled to form group mean amplitudes by coherently averaging the individual observer mean response vectors.
To estimate standard errors of the group means, we determined the magnitude of the projection of each observer's response vector onto the group mean vector (i.e. we multiplied each individual response vector amplitude by the cosine of the phase difference between it and the mean vector; Hou, Pettet & Norcia, 2008)). The magnitudes of these projections were then used to compute the mean amplitude and standard error for each condition. The mean of these projected amplitudes is the same as the amplitude of vector mean The projection procedure is useful in that it preserves a degree phase sensitivity in the analysis at the within group level that an analysis based purely on the response magnitudes does not. Although not essential for the analysis presented here, this procedure produces error estimates that more closely follow the normal distribution and are thus better suited for ANOVA/MANOVA procedures. The projection procedure also avoids the assumption of independence of the real and imaginary components that underlies the T2circ statistic that we have used previously for group errors (Norcia, Pei, Bonneh, Hou, Sampath & Pettet, 2005). T2circ was developed for the analysis of individual observer data where this assumption holds (Victor & Mast, 1991). In group data, individual differences in response magnitude create correlations between the real and imaginary coefficients that are inconsistent with the assumptions of T2circ. Such correlations create an elliptical cloud of dispersion, whose principal axes may be in any orientation with respect to the orientation (i.e., phase) of the mean vector. By projecting the individual data vectors onto the mean, we more directly assess how this dispersion relates to the amplitude of the mean, which is the parameter that we fit across conditions.
Results
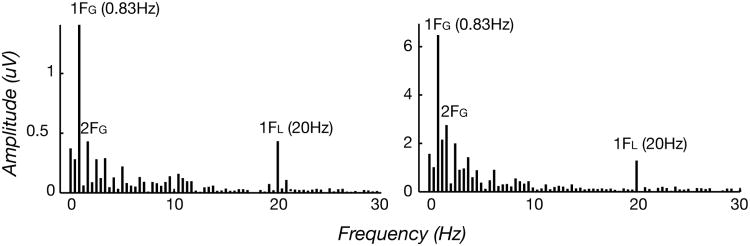
Examples of the adult and infant response spectra for coherent/incoherent motion alternations are shown in Figure 3. The spectrum was calculated via a discrete Fourier transform and represents the coherent average of data from the 14 adult observers in the left panel and 12 infants in the right panel. The dot-update rate was 20 Hz, and there is a corresponding spike in the response spectrum at that frequency (1FL). There are also prominent responses at the first few harmonics of the global-update frequency (1FG, 2FG, etc.). The infant response included robust activity at the dot update rate (1FL) and the first harmonic of the global update rate (1FG).
Figure 3.
Group average VEP amplitude spectra for the coherent motion onset/offset response of 14 adults (left panel) and 12 infants (right panel) at 3.1 deg/s (9.3 arc min)-. Coherent motion onset/offset occurred at 0.83 Hz, and there were prominent responses at the first few harmonics of the global update frequency (1FG, 2FG, etc.). The dot-update rate was 20 Hz, and there was a corresponding spike in the response spectrum at that frequency (1FL).
Responses at any harmonic of the global-update rate (nFG) are coherent motion responses by nature of the design of the stimuli: only mechanisms that can distinguish coherent motion from random motion will differentiate the two stimulus states and produce a response locked to changes in coherent motion. We can also make a distinction between the odd and even harmonic responses based on symmetry considerations. Responses at the odd harmonics (e.g., 1FG) reflect activity that is different after a transition from random motion to coherent motion (coherent motion onset) versus a transition from coherent motion to random motion (coherent motion offset). Responses at the even harmonics (e.g., 2FG) reflect responses of mechanisms that can detect presence of a transition from coherent to random or vice versa, but not the direction of the transition.
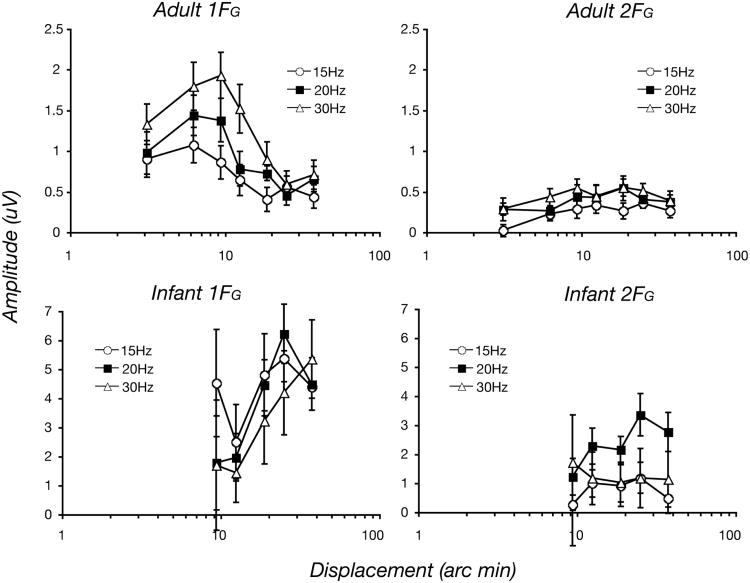
Figure 4 plots the projected magnitude of the first (1FG) and second (2FG) harmonic coherent motion responses as a function displacement at Oz for adults (top row) and infants (bottom row). There are several features of the raw data that are of note. First, the adult peak magnitudes are smaller than those of infants at all dot update rates (note the difference in scales on the ordinate). The peak 1FG magnitudes of the adult responses were largest for the 30 Hz dot-update rate, followed by the 20 Hz and 15 Hz rates at the first harmonic, but were more similar at the second harmonic. The infants had similar maximal magnitudes over the three dot-update rates at the first harmonic, but the response for the 20 Hz update rate was larger than at the 15 or 30 Hz rates at the second harmonic. The 2FG responses of the adults are much smaller than their 1FG responses, are broadly tuned and are of similar magnitude across the dot-date rates. Infant 2FG responses were robust only at the 20 Hz update rate. The remainder of the analysis focuses on the 1FG and 1FL responses, as these could be compared in a consistent fashion between infant and adult participants.
Figure 4.
Group average projected response amplitudes at 1FG and 2FG for adults (top panels) and infants (bottom panels) measured at Oz. Adult 1FG responses are largest for the 30 Hz update rate and peak at or below 10 arc min displacements. Infant 1FG responses peak at larger displacements and are more similar in magnitude at the three dot-update rates. The 2FG responses of the adults are much smaller, are broadly tuned and are of similar magnitude across the dot- update rates. Infant 2FG responses were robust only at the 20 Hz update rate. Symbol coding for the different dot-updates is shown in the inset.
Infant responses are maximal at larger displacements
The first harmonic responses of the adults were maximal for 8 to 10 arc min displacements at the Oz derivation (Figure 4, upper left panel). In contrast, the infant responses were largest at 20-40 arc min displacements at each of the three dot-update rates. As noted above, the second harmonic responses were too unreliable in the infants to make detailed comparisons of the tuning of this response component.
Speed versus displacement tuning
This experiment was designed to measure VEP amplitude as a function of two parameters --- the spatial displacement of the dots and the rate at which the dot positions were updated. These two parameters jointly determine the speed of coherent motion. By proper choice of the parameters, we were able to measure responses at a common set of speeds or a common set of displacements (see Figure 2) and we will present tuning functions in terms of both displacement and speed in an effort to determine which parameter controls the response tuning in adults and infants. The adult tuning functions were similar at O1, Oz and O2 derivations, but different at PO7 or PO8. The tuning functions were similar at PO7 and PO8 as they were symmetric lateral derivations, and we have thus chosen data from the Oz and PO8 derivations for comparison.
Because we were primarily interested in the shapes of the tuning functions at each dot-update rate, we normalized the adult response magnitudes within a dot-update rate by each observer's maximal amplitude at that rate. The maximum amplitudes differed by a factor two across the different update rates in the adults. Infant maxima were similar across update rates and we did not normalize them. We then tested for speed versus displacement tuning by comparing the shapes of the response functions when plotted on displacement or speed axes.
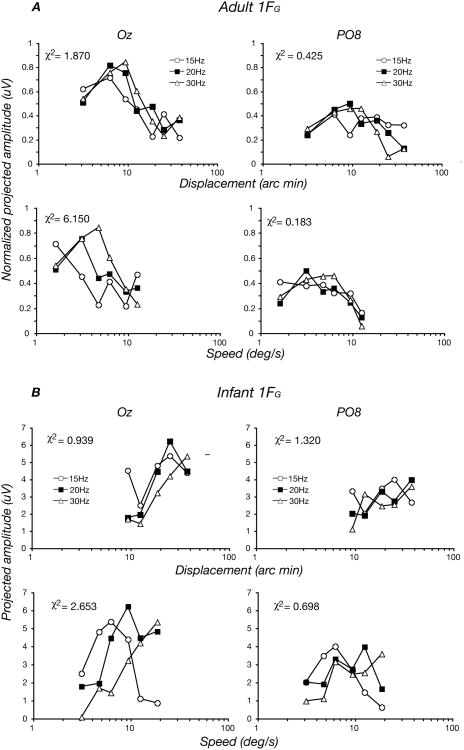
At the first harmonic of the global-update rate, the adult tuning functions largely superimpose when plotted on a displacement axis at Oz, but are better matched to each other when plotted as a function of speed at PO8, which is over right lateral cortex. These comparisons of the data are shown in Figure 5A. To determine whether displacement or speed tuning better described the data, we calculated the chi-square per degree of freedom of the individual datum points versus their respective means for the two ways of scaling the data and found lower values (better fits) for displacement at Oz and for speed at PO8. The chi-square per degree of freedom values are presented as inserts in each panel. Because the data are correlated within observers, we do not report the p-values for the fits as the effective number of degrees of freedom is biased by the within-observer correlations. This analysis suggests that responses in the early visual areas of calcarine cortex are tuned for displacement but that responses in lateral cortical areas, perhaps including the motion complex centered on the human homolog of macaque MT are tuned for speed.
Figure 5.
Global response (1F1) tuning functions for adults (A) and for infants (B) on a displacement scale (top) or on a speed scale (bottom) for Oz (left panels) and PO8 (right panels). The adult data at Oz are better described as being displacement tuned, while those at PO8 are better described as speed tuned. Fit parameters and symbol coding are shown in the insets. Infant responses are best described as being tuned for displacement at Oz, where maximal amplitudes also occur at larger displacements than in the adults.
The same analysis was applied to the infant data and is shown in Figure 5B. Because the infant response maxima were similar across conditions, we did not need to normalize them for the fitting analysis. At Oz, the infant response functions are more consistent with displacement tuning than speed tuning, especially when one considers the lower speed/smaller displacement side of the functions. The infant data from PO8 is quite variable and the signal is small relative to Oz, so it is difficult to make a clear distinction between speed and displacement tuning there. Normalization of the infant data produced poorer fits overall, presumably because the individual infant maximal responses were quite variable.
Spatio-temporal tuning of the dot-update response
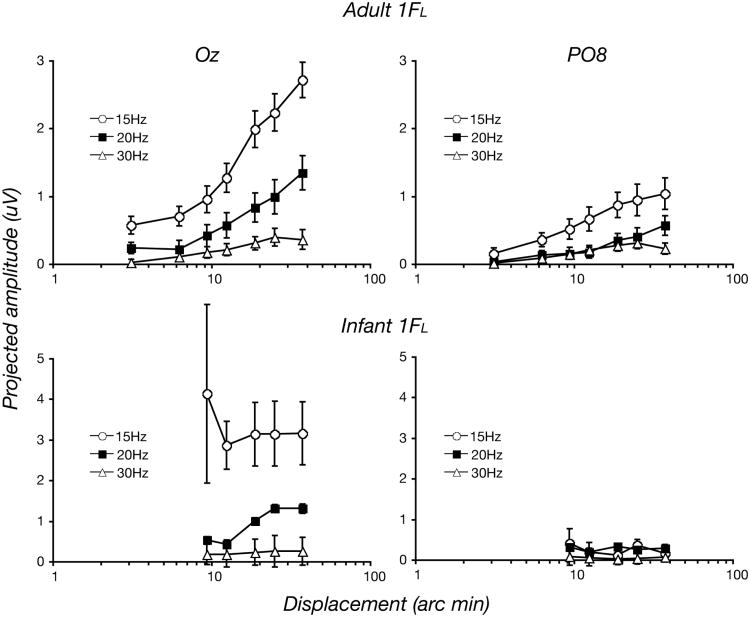
Robust responses were recorded in both infants and adults at the temporal frequency of the dot-update rate. The first harmonic component of the dot-update response (1FL) was maximal at Oz in both infants and adults. Figure 6 plots these data as projected amplitudes at Oz (left panels) and PO8 (right panels) with the adult data in the top panels and the infant data in the bottom panels. At Oz, the largest magnitude responses were recorded at 15 Hz, decreasing at 20 Hz and further decreasing at 30 Hz in both infant and adults. The adult responses increase systematically with increasing displacement at both electrode sites. The infant responses decrease from about 4.5 microvolts for the 10 arc min displacement to a constant level of 3 microvolts at the larger displacement. Adult responses are increasing over this range. Infant responses at 20 Hz show an increase in response over the same range, while at 30 Hz, the infant responses are at the noise level. The response amplitudes decreased at the lateral electrodes, especially in the infants (data from PO8 is shown). Infant and adult peak response magnitudes are very similar at each of the three dot update rates at Oz. Response amplitudes decline at PO8, relative to Oz, especially in the infant. Maximal responses in the infants are smaller by about a factor of ten at PO8 relative to Oz versus a factor of three in the adults. Overall, the infant dot-update responses were more similar to those of the adults than were the responses driven by the changes in coherence.
Figure 6.
Spatio-temporal tuning of the dot-update responses at Oz (left panels) and PO8 (right panels) across observers. Infants (bottom panels) and adults (top panels) showed similar local motion sensitivity in terms of spatio-temporal tuning at Oz, with the highest response for 15 Hz dot-update rate, followed by 20 Hz, followed by 30 Hz. The responses in adults are reduced at lateral electrode sites by about a factor of three while responses in infants diminished by a factor of about 10.
Discussion
Cortical responses to coherent motion depend parametrically on the speed/displacement of the patterns in both infants and adults. At the first harmonic of the global motion update rate, the adult evoked response is tuned for displacement at the occipital midline, but is tuned for speed at lateral electrodes. Infant responses were tuned for displacement at Oz but were weak and poorly tuned at lateral electrode sites. The occipital midline electrode preferentially samples visual areas in and around the calcarine fissure and dorsally towards the vertex reference, while the lateral derivations receive more of a contribution from motion sensitive areas in and around the human homologue of macaque MT. From our data it is tempting to conclude that speed sensitivity in adults is first elaborated in hMT+, but conclusive evidence would require a formal analysis of sources with high-density recordings.
The pattern of results we observe at lateral recording sites versus the occipital pole in adults is broadly consistent with known properties of single cells in cortical areas MT and V1 of the macaque, respectively. Simple cells in V1 have separable tuning for spatial and temporal frequency, but a sizable number of V1 complex cells show a degree of inseparability and thus speed tuning (Priebe, Lisberger & Movshon, 2006). The spatio-temporal tuning for single gratings in MT is similar to that of complex cells, e.g. moderately inseparable (Priebe, Cassanello & Lisberger, 2003). However, when stimuli that contain a range of spatial frequencies are presented, MT cells are more specifically tuned for speed than are V1 complex cells or simple cells (Priebe et al., 2003, Priebe et al., 2006). Our stimuli contain a range of spatial frequencies and would thus be expected to elicit a largely speed tuned response from the human homologue of MT. It is thus plausible that the transition from displacement to speed tuning seen between medial and lateral electrode sites in adults in the present study reflects a speed tuned population of neurons in MT versus stronger displacement tuning in the populations that provide its inputs.
Previous psychophysical studies of coherence thresholds have not found them to be tuned for speed, but rather for displacement (Baker & Braddick, 1985, Kiorpes & Movshon, 2004). The present measurements were based on highly supra-threshold stimuli that may recruit a different population of cells than the one that determines the perceptual coherence threshold. Adult second harmonic responses are smaller than the first harmonic responses, are not preferentially tuned for speed or displacement and are maximal at larger displacements than are the first harmonic responses. In adults, the first and second harmonics are thus likely to be generated by different processes. The infant second harmonic data are too weak to draw a similarly strong conclusion.
Maximal first harmonic responses are recorded at substantially larger displacements/higher speeds in infants than in adults. A similar sensitivity pattern has been observed previously in a behavioral study in macaques (Kiorpes & Movshon, 2004). Visual deprivation has its greatest effects on low velocities both with the VEP (Hou, Pettet & Norcia, 2008) and psychophysically (Hess & Anderson, 1993, Kiorpes, Tang & Movshon, 2006, Steinman, Levi & McKee, 1988), further suggesting that maturation proceeds from coarse space and time scales to finer ones as suggested by Kiorpes and colleagues (Kiorpes & Movshon, 2004, Kiorpes et al., 2006). This developmental pattern differs from that observed using the direction reversal VEP (Wattam-Bell, 1991). Wattam-Bell's direction reversal paradigm assays direction-specific responses by recording responses evoked by changes in direction of low spatial frequency square-wave gratings, rather than the onset/offset of coherent dot motion. The large differences in stimuli between our study and his may result in the different order of development of high and low speeds. The present results are consistent with a large body of behavioral data indicating that young infants are sensitive to coherent motion (Braddick et al., 2003, Gilmore, Hou, Pettet & Norcia, 2007). Behavioral sensitivity to coherent motion improves monotonically between 1 and 8 deg/s (Banton & Bertenthal, 1996). We observe an increase in evoked response amplitude over this range.
Local motion/flicker sensitivity
Responses measured at the dot-update rate comprise contributions of local motion and temporal contrast mechanisms in an unknown mixture. These responses have nearly adult-like spatio-temporal tuning at 4-6 months. Maximal responses occur over the occipital midline where they decline in amplitude at a similar rate as a function of the dot-update rate. The fall-off in magnitude of the response at lateral electrodes is much steeper in the infants than in the adults. This could due to a passive effect of the lower conductivity skull of the adults, or it could reflect a genuine immaturity of lateral cortical areas. Formal source analysis in both infants and adults would be needed to decide which explanation is correct. More direct evidence for an immaturity in developing extra-striate cortex comes from a recent fMRI study (Kourtzi, Augath, Logothetis, Movshon & Kiorpes, 2006). Kourtzi and co-workers studied the development of visual BOLD activation longitudinally in two infant monkeys aged between 14 and 80 weeks. The youngest and oldest infant monkeys showed the same visual activation in V1 as adult animals. However, visual activation in extra-striate area MT/V5, which has been strongly implicated in the processing of coherent motion was not evident in the youngest animals, but became more adult-like in the older animals.
Limitations of the present study
Coherent motion displays are designed to selectively stimulate specific populations and, as a result, responses to these displays are small, even in adults. The small intrinsic magnitudes of these responses, combined with the short attention span of infants, makes detailed parametric studies, such as this one challenging.
Due to the limited attention spans of infants we were not able to measure complete tuning functions in a within-observer design. Instead, we used a combination of within- and between-observer designs that are difficult to analyze with traditional statistical methods such as ANOVA/MANOVA. We are thus unable to make precise statements about the statistical significance of some of the main and interaction effects of our parametric design. We relied instead on goodness of fit metrics for different scaling relationships. Here again the analysis needed to be qualitative due to the complex correlation structure in the data. Finally, some of the infant responses were very close to the experimental noise level and we have been cautious in making strong conclusions, particularly about the nature of tuning at lateral electrode sites in infants. Nonetheless, several basic differences in the coherent motion response between infants and adults are apparent, as discussed above.
We have used fixation prompts in an attempt to maximize the number of trials we could obtain and to control accommodation and fixation. However, coherent motion responses are reduced when adult observers are given a distractor task to perform (Rees, Frith & Lavie, 1997). A similar effect may occur in infants, reducing the response amplitudes.
The infant responses were more variable than those of the adults. Previous work on the pattern reversal VEP (Norcia & Tyler, 1985) has suggested that between-observer variability in infants is dominated by individual differences rather than measurement error. It would be of interest to measure test-retest reliability for the coherent motion response to see if the same holds true for this measure, or if the variability is simply due to the lower signal to noise ratio of this response measure.
Taken together, our results suggest that while infants are sensitive to coherent motion displays, they display a number of significant immaturities, the most important of which are a preference for larger displacements/speeds and an apparent lack of speed tuning. These immaturities do not seem to be due to an immature pattern of response to the dots themselves, as these are quite adult-like. Rather, they are likely to be due to immaturities at higher, more integrative stages of processing.
Acknowledgments
Supported by EY015790 (AMN) and NSF BCS 00-92452 (ROG). We thank all the parents and infants who participated in this study and Margaret McGovern for her assistance in conducting the infant experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aspell JE, Tanskanen T, Hurlbert AC. Neuromagnetic correlates of visual motion coherence. Eur J Neurosci. 2005;22(11):2937–2945. doi: 10.1111/j.1460-9568.2005.04473.x. [DOI] [PubMed] [Google Scholar]
- Atkinson J. Oxford Psychology Series. Vol. 32. Oxford University Press; 2000. The developing visual brain. [Google Scholar]
- Baker CL, Jr, Braddick OJ. Temporal properties of the short-range process in apparent motion. Perception. 1985;14(2):181–192. doi: 10.1068/p140181. [DOI] [PubMed] [Google Scholar]
- Banton T, Bertenthal BI. Infants' sensitivity to uniform motion. Vision Res. 1996;36(11):1633–1640. doi: 10.1016/0042-6989(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Banton T, Bertenthal BI, Seaks J. Infants' sensitivity to statistical distributions of motion direction and speed. Vision Res. 1999;39(20):3417–3430. doi: 10.1016/s0042-6989(99)00100-5. [DOI] [PubMed] [Google Scholar]
- Birch EE, Fawcett S, Stager D. Co-development of VEP motion response and binocular vision in normal infants and infantile esotropes. Invest Ophthalmol Vis Sci. 2000;41(7):1719–1723. [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41(13):1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick O, Birtles D, Wattam-Bell J, Atkinson J. Motion- and orientation-specfic cortical responses in infancy. Vision Res. 2005;45(25-26):3169–3179. doi: 10.1016/j.visres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O'Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30(1):61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O'Brien JM, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Curr Biol. 2000;10(12):731–734. doi: 10.1016/s0960-9822(00)00540-6. [DOI] [PubMed] [Google Scholar]
- Chino YM, Smith EL, 3rd, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci. 1997;17(1):296–307. doi: 10.1523/JNEUROSCI.17-01-00296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Berman N, Hein A. Cats reared in stroboscopic illumination: effects on receptive fields in visual cortex. Proc Natl Acad Sci U S A. 1973;70(5):1353–1354. doi: 10.1073/pnas.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Chernenko G. Abolition of direction selectivity in the visual cortex of the cat. Science. 1976;193(4252):504–505. doi: 10.1126/science.941025. [DOI] [PubMed] [Google Scholar]
- Distler C, Vital-Durand F, Korte R, Korbmacher H, Hoffmann KP. Development of the optokinetic system in macaque monkeys. Vision Res. 1999;39(23):3909–3919. doi: 10.1016/s0042-6989(99)00122-4. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. J Neurosci. 1995;15(7 Pt 2):5192–5208. doi: 10.1523/JNEUROSCI.15-07-05192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Dirks M, Maurer D, Ledgeway T, Guillemot JP, Lepore F. Putting order into the development of sensitivity to global motion. Vision Res. 2004;44(20):2403–2411. doi: 10.1016/j.visres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brar S, Brent HP. Better perception of global motion after monocular than after binocular deprivation. Vision Res. 2002;42(2):169–179. doi: 10.1016/s0042-6989(01)00278-4. [DOI] [PubMed] [Google Scholar]
- Gilmore RO, Hou C, Pettet MW, Norcia AM. Development of cortical responses to optic flow. Vis Neurosci. 2007;24(6):845–856. doi: 10.1017/S0952523807070769. [DOI] [PubMed] [Google Scholar]
- Goossens J, Dukelow SP, Menon RS, Vilis T, van den Berg AV. Representation of head-centric flow in the human motion complex. J Neurosci. 2006;26(21):5616–5627. doi: 10.1523/JNEUROSCI.0730-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002;13(6):843–847. doi: 10.1097/00001756-200205070-00021. [DOI] [PubMed] [Google Scholar]
- Handel B, Lutzenberger W, Thier P, Haarmeier T. Opposite dependencies on visual motion coherence in human area MT+ and early visual cortex. Cereb Cortex. 2007;17(7):1542–1549. doi: 10.1093/cercor/bhl063. [DOI] [PubMed] [Google Scholar]
- Hess RF, Anderson SJ. Motion sensitivity and spatial undersampling in amblyopia. Vision Res. 1993;33(7):881–896. doi: 10.1016/0042-6989(93)90071-4. [DOI] [PubMed] [Google Scholar]
- Heuer HW, Britten KH. Optic flow signals in extrastriate area MST: comparison of perceptual and neuronal sensitivity. J Neurophysiol. 2004;91(3):1314–1326. doi: 10.1152/jn.00637.2003. [DOI] [PubMed] [Google Scholar]
- Hou C, Pettet MW, Norcia AM. Abnormalities of coherent motion processing in strabismic amblyopia: Visual-evoked potential measurements. J Vis. 2008;8(4):2.1–12. doi: 10.1167/8.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB. Strobe rearing reduces direction selectivity in area 17 by altering spatiotemporal receptive-field structure. J Neurophysiol. 1998;80(6):2991–3004. doi: 10.1152/jn.1998.80.6.2991. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB, Feidler JC. Strobe rearing prevents the convergence of inputs with different response timings onto area 17 simple cells. J Neurophysiol. 1998;80(6):3005–3020. doi: 10.1152/jn.1998.80.6.3005. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Vis Neurosci. 2004;21(6):851–859. doi: 10.1017/S0952523804216054. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Tang C, Movshon JA. Sensitivity to visual motion in amblyopic macaque monkeys. Vis Neurosci. 2006;23(2):247–256. doi: 10.1017/S0952523806232097. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Augath M, Logothetis NK, Movshon JA, Kiorpes L. Development of visually evoked cortical activity in infant macaque monkeys studied longitudinally with fMRI. Magn Reson Imaging. 2006;24(4):359–366. doi: 10.1016/j.mri.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Koyama S, Sasaki Y, Andersen GJ, Tootell RB, Matsuura M, Watanabe T. Separate processing of different global-motion structures in visual cortex is revealed by FMRI. Curr Biol. 2005;15(22):2027–2032. doi: 10.1016/j.cub.2005.10.069. [DOI] [PubMed] [Google Scholar]
- Kremenitzer JP, Vaughan HG, Jr, Kurtzberg D, Dowling K. Smooth-pursuit eye movements in the newborn infant. Child Dev. 1979;50(2):442–448. [PubMed] [Google Scholar]
- Lam K, Kaneoke Y, Gunji A, Yamasaki H, Matsumoto E, Naito T, Kakigi R. Magnetic response of human extrastriate cortex in the detection of coherent and incoherent motion. Neuroscience. 2000;97(1):1–10. doi: 10.1016/s0306-4522(00)00037-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9(5):676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456(7224):952–956. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 2000;3(12):1322–1328. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- Naegele JR, Held R. The postnatal development of monocular optokinetic nystagmus in infants. Vision Res. 1982;22(3):341–346. doi: 10.1016/0042-6989(82)90149-3. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kashii S, Nagamine T, Matsui Y, Hashimoto T, Honda Y, Shibasaki H. Human V5 demonstrated by magnetoencephalography using random dot kinematograms of different coherence levels. Neurosci Res. 2003;46(4):423–433. doi: 10.1016/s0168-0102(03)00119-6. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedeggen M, Wist ER. Characteristics of visual evoked potentials generated by motion coherence onset. Brain Res Cogn Brain Res. 1999;8(2):95–105. doi: 10.1016/s0926-6410(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Garcia H, Humphry R, Holmes A, Hamer RD, Orel-Bixler D. Anomalous motion VEPs in infants and in infantile esotropia. Invest Ophthalmol Vis Sci. 1991;32(2):436–439. [PubMed] [Google Scholar]
- Norcia AM, Pei F, Bonneh Y, Hou C, Sampath V, Pettet MW. Development of sensitivity to texture and contour information in the human infant. J Cogn Neurosci. 2005;17(4):569–579. doi: 10.1162/0898929053467596. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW. Infant VEP acuity measurements: analysis of individual differences and measurement error. Electroencephalogr Clin Neurophysiol. 1985;61(5):359–369. doi: 10.1016/0013-4694(85)91026-0. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guerit JM, Hinrichs H, Ikeda A, Luccas FJ, Rappelsburger P. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1998;106(3):259–261. doi: 10.1016/s0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Finocchio DV, Ong L, Fuchs AF. Smooth pursuit in 1- to 4-month-old human infants. Vision Res. 1997;37(21):3009–3020. doi: 10.1016/s0042-6989(97)00107-7. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Cassanello CR, Lisberger SG. The neural representation of speed in macaque area MT/V5. J Neurosci. 2003;23(13):5650–5661. doi: 10.1523/JNEUROSCI.23-13-05650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG, Movshon JA. Tuning for spatiotemporal frequency and speed in directionally selective neurons of macaque striate cortex. J Neurosci. 2006;26(11):2941–2950. doi: 10.1523/JNEUROSCI.3936-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278(5343):1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rosander K. Visual tracking and its relationship to cortical development. Prog Brain Res. 2007;164:105–122. doi: 10.1016/S0079-6123(07)64006-0. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Somers DC, Dale AM, Tootell RB. Functional MRI studies of human visual motion perception: texture, luminance, attention and after-effects. Cereb Cortex. 2003;13(4):340–349. doi: 10.1093/cercor/13.4.340. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Treue S, Andersen RA. The response of neurons in areas V1 and MT of the alert rhesus monkey to moving random dot patterns. Exp Brain Res. 1992;88(2):389–400. doi: 10.1007/BF02259114. [DOI] [PubMed] [Google Scholar]
- Steinman S, Levi DM, McKee SP. Temporal asynchrony and velocity discrimination in the amblyopic visual system, Clinical Vision Sciences 2:265-276. Clinical Vision Sciences. 1988;2:265–276. [Google Scholar]
- Tanaka K, Saito H. Analysis of motion of the visual field by direction, expansion/contraction, and rotation cells clustered in the dorsal part of the medial superior temporal area of the macaque monkey. J Neurophysiol. 1989;62(3):626–641. doi: 10.1152/jn.1989.62.3.626. [DOI] [PubMed] [Google Scholar]
- Tang Y, Norcia AM. An adaptive filter for steady-state evoked responses. Electroencephalogr Clin Neurophysiol. 1995;96(3):268–277. doi: 10.1016/0168-5597(94)00309-3. [DOI] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Volkmann FC, Dobson MV. Infant responses of ocular fixation to moving visual stimuli. J Exp Child Psychol. 1976;22(1):86–99. doi: 10.1016/0022-0965(76)90092-8. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Bi H, Zhang B, Sakai E, Mori T, Harwerth RS, Smith EL, 3rd, Chino YM. Directional bias of neurons in V1 and V2 of strabismic monkeys: temporal-to-nasal asymmetry? Invest Ophthalmol Vis Sci. 2005;46(10):3899–3905. doi: 10.1167/iovs.05-0563. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Development of motion-specific cortical responses in infancy. Vision Res. 1991;31(2):287–297. doi: 10.1016/0042-6989(91)90119-p. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Coherence thresholds for discrimination of motion direction in infants. Vision Res. 1994;34(7):877–883. doi: 10.1016/0042-6989(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Visual motion processing in one-month-old infants: preferential looking experiments. Vision Res. 1996;36(11):1671–1677. doi: 10.1016/0042-6989(95)00236-7. [DOI] [PubMed] [Google Scholar]