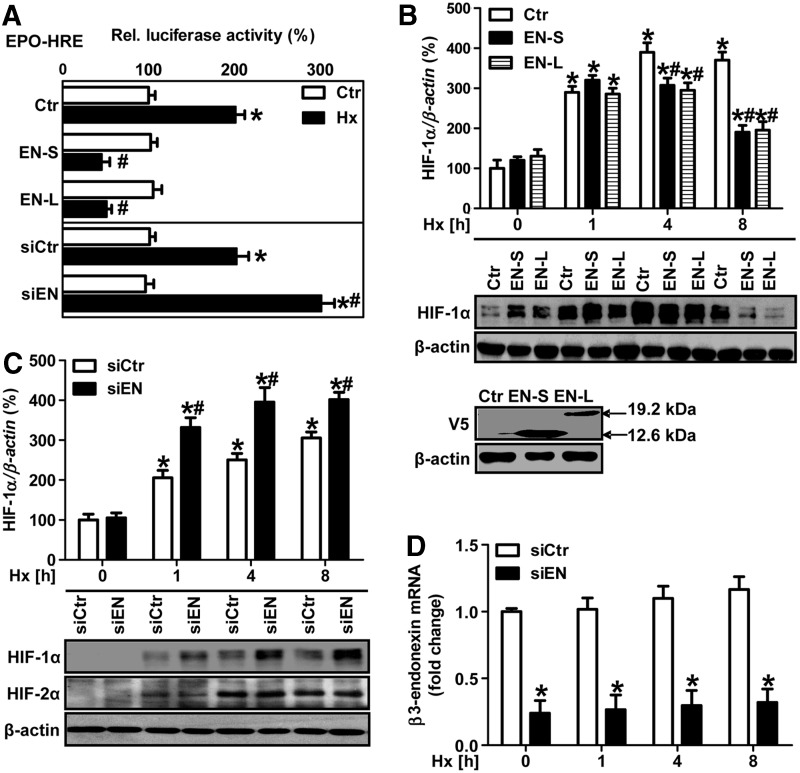
FIG. 3.
β3-endonexin decreases HIF activity and HIF-1α protein levels under hypoxia. (A) HMEC-1 cells were co-transfected with a luciferase construct driven by three hypoxia-response elements (HRE) from the erythropoietin gene (EPO-HRE) and expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) or with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr). HMEC-1 cells were exposed to hypoxia (1% oxygen, Hx) for 8 h, and luciferase assay was performed. Normoxic controls (Ctr) were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). (B) HMEC-1 cells were transfected with expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) and exposed to hypoxia for increasing time points or cultivated under normoxia (0 h). Western blot analyses were performed with antibodies against HIF-1α and β-actin. Normoxic controls (Ctr, 0 h) were set equal to 100% (n=3, *p<0.05 vs. Ctr 0 h; #p<0.05 vs. Ctr 1, 4 or 8 h). Expression of β3-endonexin short (EN-S) or long (EN-L) constructs was validated by Western blotting using a V5 antibody. (C, D) HMEC-1 cells were transfected with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr) and exposed to hypoxia for 1, 4, and 8 h or cultivated under normoxia (0 h). (C) Western blot analyses were performed with antibodies against HIF-1α and HIF-2α. β-actin levels served as a loading control. HIF-1α levels at normoxia (siCtr, 0 h) were set equal to 100% (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h). (D) RT-qPCR analyses were performed with primers amplifying human β3-endonexin and 18S rRNA for normalization. Control levels at normoxia (siCtr, 0 h) were set equal to 1 (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h).