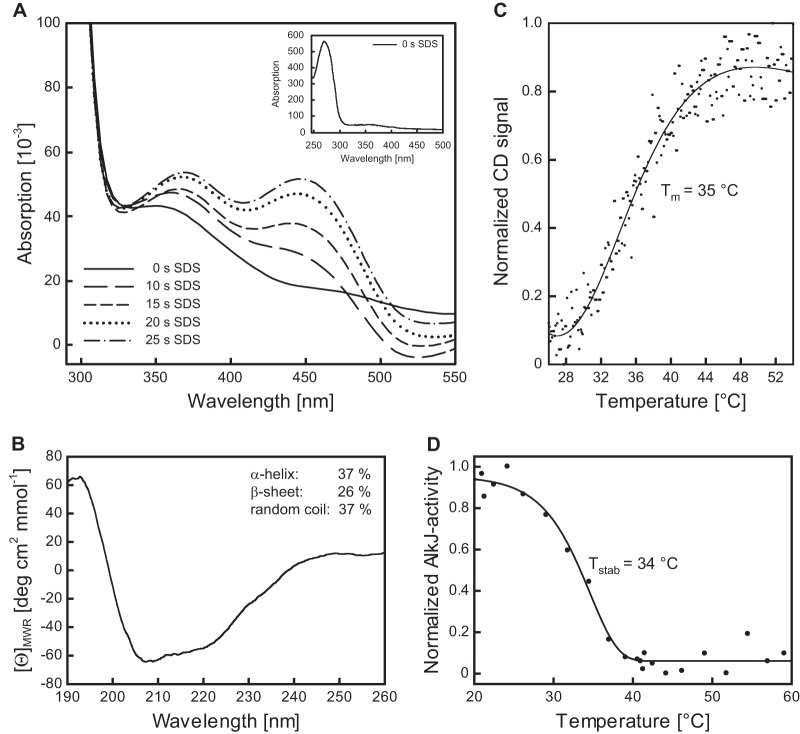
FIG 3.
Spectroscopic characterization of the recombinant AlkJ holoenzyme and thermal stability analysis. (A) Time-dependent cofactor release from AlkJ upon enzyme denaturation. Reoxidation of the liberated FADH2 from a 10 μM solution of IMAC-purified AlkJ after addition of 0.2% (wt/vol) SDS was monitored at different time points using a diode array spectrophotometer. During the progress of this reaction, two absorption maxima emerged, at around 370 and 450 nm, presumably reflecting the oxidation of the released coenzyme FADH2 by dissolved oxygen. The inset depicts the UV-visible spectrum of the initial IMAC-purified AlkJ, which lacks the characteristic peaks for oxidized FAD but reveals the typical prominent absorption maximum at 280 nm for the aromatic amino acid side chains of the enzyme. This peak is neighbored by a broad shoulder extending to longer wavelengths, which is indicative of FADH2. (B) Far-UV circular dichroism (CD) spectrum of IMAC-purified recombinant AlkJ. The presence of both α-helical and β-sheet secondary structure can be deduced from the shape of the broad negative band in the region of 210 to 220 nm. Estimation of the secondary structure content was performed with the K2D algorithm available at the Dichroweb server (66). (C) Quasireversible thermal denaturation at pH 7.5 was monitored in a CD spectropolarimeter at 215 nm while heating at a constant rate of 60 K/h. Refolding could not be measured in this assay due to the formation of protein aggregates upon denaturation. (D) Thermally induced irreversible denaturation at pH 8.0 was assessed after 60 min of incubation at various temperatures by determining the residual enzymatic activity toward 0.1 mM 1-octanol (with 0.2 mM PMS and 0.1 mM DCPIP) at 30°C.