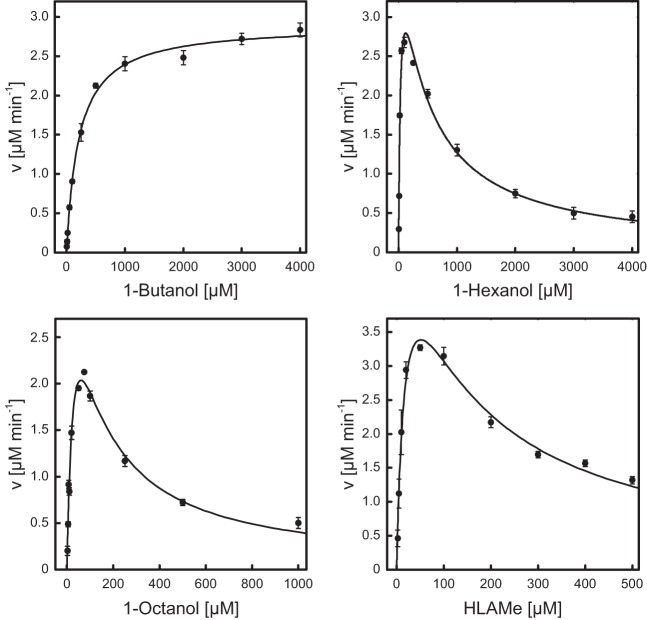
FIG 4.
Catalytic activity of AlkJ on different alcohol substrates. The kinetic features of the purified recombinant enzyme toward the aliphatic alcohols 1-butanol, 1-hexanol, and 1-octanol as well as HLAMe, an industrially relevant long-chain alcohol derivative, were investigated using a 0.2 μM solution of the enzyme in 50 mM NaPi (pH 7.5) and PMS/DCPIP as oxidants in microwell plates. The redox state of the terminal electron acceptor DCPIP was monitored in situ at 600 nm. The initial reaction velocities measured with various alcohol concentrations were subjected to curve fitting according to Michaelis-Menten kinetics, taking into account uncompetitive substrate inhibition for the three alcohols with longer hydrocarbon chains.