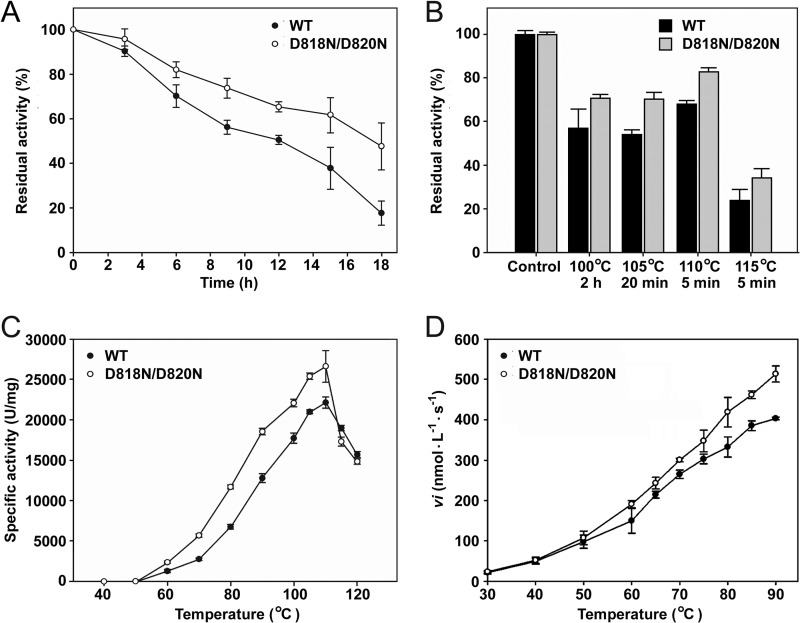
FIG 5.
Properties of the WT and the D818N/D820N mutant. (A and B) Heat inactivation of the enzymes. The purified samples of the enzymes (5.0 μg/ml) in buffer A were incubated at 95°C (A) or at 100 to 115°C (B) for the time intervals indicated and then subjected to an azocaseinolytic activity assay. The residual activity was calculated on the basis of the definition of the activity of the non-heat-treated sample (Control) as 100%. (C) Temperature dependence of azocaseinolytic activity. Activity assays were performed in buffer A for 10 min at the indicated temperatures using 0.5% azocasein as the substrate. (D) Temperature dependence of the activity toward suc-AAPK-pNA. The initial velocities (vi) of suc-AAPK-pNA (0.5 mM) hydrolysis catalyzed by the enzymes (0.1 μg/ml) were measured in buffer A over a temperature range of 30 to 90°C. The values are expressed as means ± SDs (bars) of the results of three independent experiments.