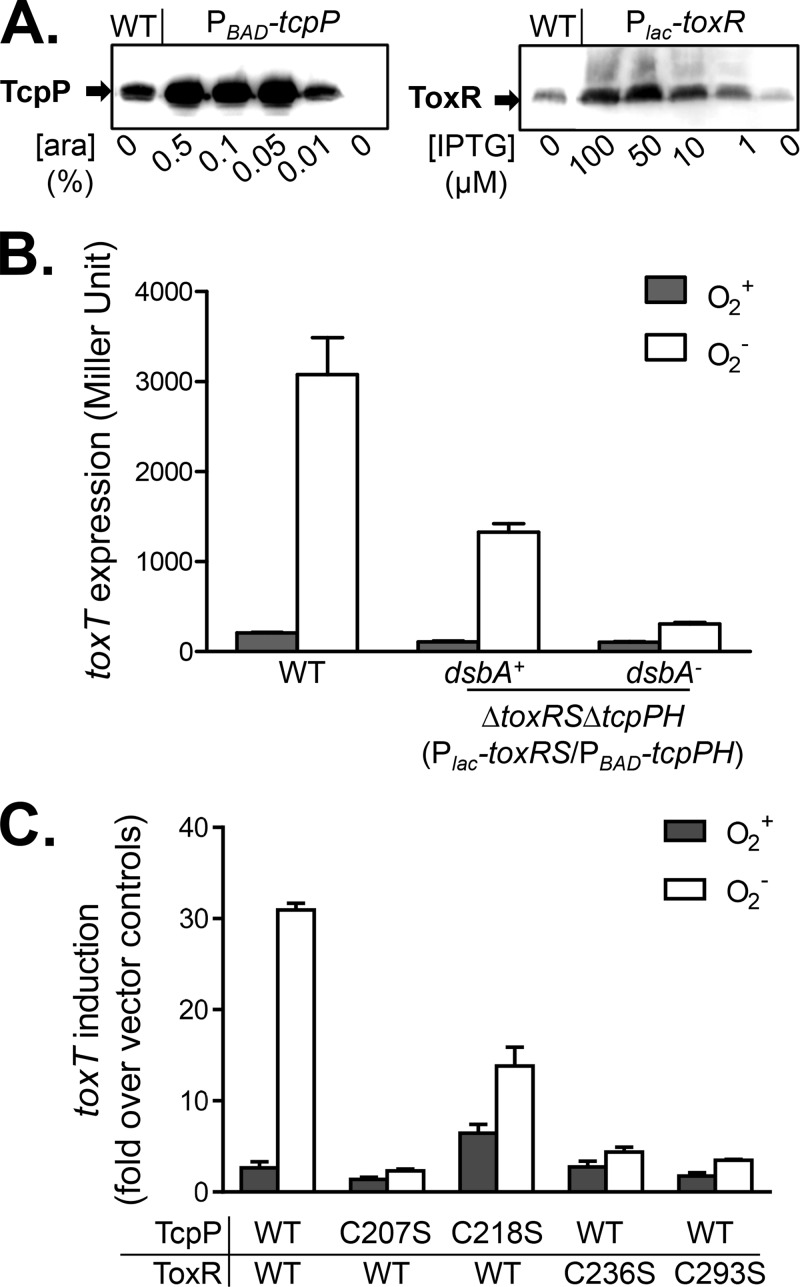
FIG 4.
Effects of oxygen on the ToxR-TcpP interaction and virulence expression in V. cholerae. (A) Concentrations of inducers needed to express TcpP and ToxR to wild-type (WT) levels. Cultures of wild-type or ΔtoxRS ΔtcpPH mutant V. cholerae containing PBAD-tcpPH and Plac-toxRS were grown in AKI medium in the presence of various amounts of arabinose (ara) or IPTG without shaking at 37°C to an OD600 of ∼0.3. The cell pellets were subjected to Western blot assays with anti-TcpP (left panel) and anti-ToxR (right panel) antibodies. (B) Analysis of ToxR-TcpP interactions and activation of toxT. Wild-type V. cholerae (PtoxT-lacZ), a ΔtoxRS ΔtcpPH mutant, and a ΔtoxRS ΔtcpPH ΔdsbA mutant containing PBAD-tcpPH, Plac-toxRS, and PtoxT-lacZ reporter plasmids were grown aerobically or anaerobically in AKI medium supplemented with 0.01% arabinose and 1 μM IPTG to an OD600 of ∼0.3. β-Galactosidase activity was then measured. Data represent the mean ± the standard deviation of three independent experiments. (C) Analysis of the effects of periplasmic cysteine mutations on ToxR-TcpP interactions and activation of toxT. The ΔtoxRS ΔtcpP mutant (21) containing a PBAD-tcpPH (or its cysteine→serine derivatives), Plac-toxRS (or its cysteine→serine derivative mutant forms), or PtoxT-lacZ reporter plasmid was grown aerobically or anaerobically in AKI medium supplemented with 0.01% arabinose and 1 μM IPTG to an OD600 of ∼0.3. β-Galactosidase activity was then measured. Data represent the mean ± the standard deviation of three independent experiments.