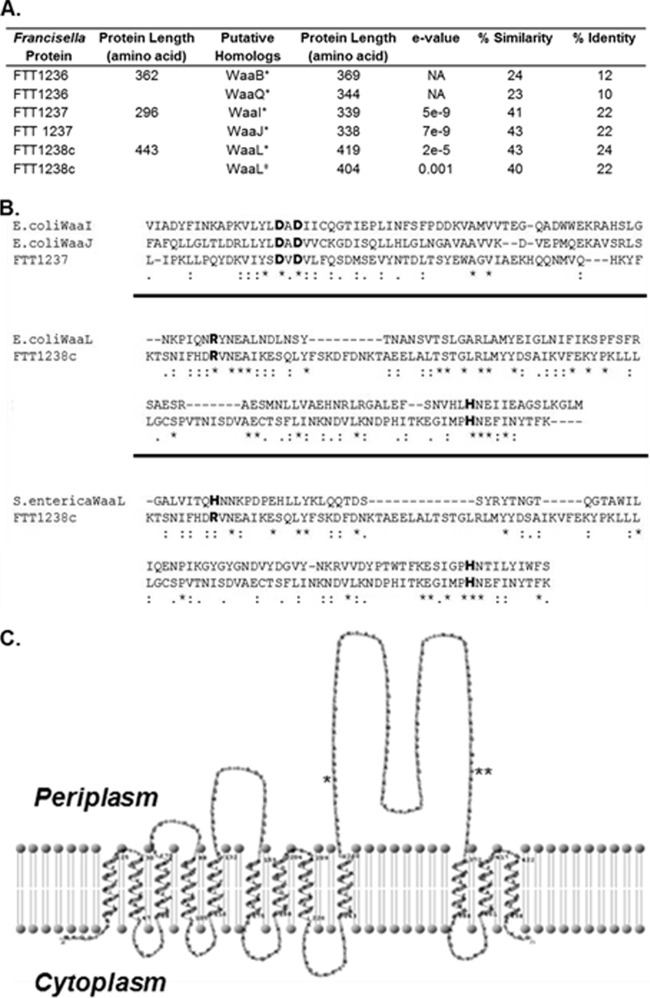
FIG 1.
Similarity of F. tularensis Waa proteins to E. coli and S. enterica proteins. (A) Francisella proteins FTT1236, FTT1237, and FTT1238c are similar to E. coli (*) and S. enterica (‡) LPS biosynthetic proteins. (B) In the protein alignments, single dots denote semiconserved residues, double dots are conserved residues, and asterisks identify exact matches of amino acid residues. Individual alignments are separated by thick black lines. The FTT1237 protein has a DxD motif (shown in bold) that is conserved in both WaaI and WaaJ of E. coli. Alignment of the FTT1238c protein to the E. coli and S. enterica WaaL proteins reveals the presence of the highly conserved arginine and histidine amino acids (in bold) that are important for the function of the protein. (C) The membrane topology map of FTT1238c shows the 12 predicted transmembrane domains as well as a large periplasmic loop containing the conserved arginine (*; R270) and histidine (**; H361) residues.