Abstract
Several animal models exist to evaluate the immunogenicity and protective efficacy of candidate Shigella vaccines. The two most widely used nonprimate models for vaccine development include a murine pulmonary challenge model and a guinea pig keratoconjunctivitis model. Nonhuman primate models exhibit clinical features and gross and microscopic colonic lesions that mimic those induced in human shigellosis. Challenge models for enterotoxigenic Escherichia coli (ETEC) and Campylobacter spp. have been successfully developed with Aotus nancymaae, and the addition of a Shigella-Aotus challenge model would facilitate the testing of combination vaccines. A series of experiments were designed to identify the dose of Shigella flexneri 2a strain 2457T that induces an attack rate of 75% in the Aotus monkey. After primary challenge, the dose required to induce an attack rate of 75% was calculated to be 1 × 1011 CFU. Shigella-specific immune responses were low after primary challenge and subsequently boosted upon rechallenge. However, preexisting immunity derived from the primary challenge was insufficient to protect against the homologous Shigella serotype. A successive study in A. nancymaae evaluated the ability of multiple oral immunizations with live-attenuated Shigella vaccine strain SC602 to protect against challenge. After three oral immunizations, animals were challenged with S. flexneri 2a 2457T. A 70% attack rate was demonstrated in control animals, whereas animals immunized with vaccine strain SC602 were protected from challenge (efficacy of 80%; P = 0.05). The overall study results indicate that the Shigella-Aotus nancymaae challenge model may be a valuable tool for evaluating vaccine efficacy and investigating immune correlates of protection.
INTRODUCTION
Shigellosis, or bacillary dysentery, resulted in more than 100,000 deaths globally in 2010, mostly in developing countries (1). Although shigellosis is considered a disease of developing countries, over 14,000 laboratory-confirmed cases are reported to occur in the United States annually (2). In the United States, Shigella infections constitute the third most common cause of gastroenteritis, after Campylobacter and Salmonella infections. Populations particularly susceptible are children in day care centers, migrant workers, travelers to developing countries, and homosexual men (3–6). The low infectious dose, the fecal-oral route of transmission, and the emergence of resistance to multiple antibiotics among Shigella isolates pose a major public health problem throughout the developing world and necessitate the development of a safe, efficacious vaccine.
There are several animal models to investigate pathogenic mechanisms utilized by Shigella spp. and to evaluate the immunogenicity and protective efficacy of candidate vaccines. The two most widely used models for vaccine development include a murine pulmonary challenge model (7), which is useful for preliminary screening of vaccine candidates, and a guinea pig keratoconjunctivitis model (8). The ability of Shigella to invade the corneal epithelium of guinea pigs and spread to contiguous cells, causing keratoconjunctivitis, provides a model system that mimics the invasive process that occurs in the mucosal epithelium. Recently, a guinea pig rectocolitis model has been described (9) that induces bloody, mucoidal stools. Adaptations to the published protocol have facilitated use of the rectocolitis model in vaccination/efficacy studies in larger and older guinea pigs (R. W. Kaminski and E. V. Oaks, unpublished data).
Nonhuman primate models also exist for shigellosis and have been used to better understand pathogenesis (10) and to evaluate vaccine immunogenicity and efficacy (11). In the rhesus monkey model, oral challenge doses are administered at levels of 1 × 1010 to 1 × 1011 CFU, and the animals are given bicarbonate solution to neutralize stomach acidity. The clinical features combined with gross and microscopic colonic lesions induced by wild-type shigellae in monkeys are similar to those induced in human shigellosis (12). The similar disease courses and pathologies of human and monkey shigellosis provide an excellent model to study shigellosis. Despite the similarities, several differences remain between the pathology associated with human and monkey shigellosis. For example, gastric mucosal lesions have been observed in rhesus monkeys after experimental or natural infection with shigellae (10), whereas in humans, lesions are limited to the colonic epithelium (13). Oral feeding of rhesus monkeys with Shigella flexneri 2a induces an inflammatory reaction in the gastric mucosa that is similar to that in the gut. The gastric lesions could be a result of the high level of bacteria (1010 CFU) needed for challenge or differences in rhesus monkey physiology compared to human physiology.
In recent years, oral challenge models have been developed in Aotus nancymaae monkeys for both Campylobacter jejuni and enterotoxigenic Escherichia coli (ETEC). Both Aotus challenge models result in reproducible attack rates of ≥70% and are characterized by colonization of the gastrointestinal tract and the induction of diarrhea (14, 15). The addition of a Shigella-Aotus challenge model would enable the testing of potential combination vaccines against the three most common enteric bacterial pathogens responsible for traveler's diarrhea.
To that end, the research described herein focuses on determining a dose of S. flexneri 2a strain 2457T that reproducibly achieved an attack rate of ≥75%. Once the challenge dose was established, the immunogenicity and protective efficacy of a well-characterized, live-attenuated Shigella flexneri 2a vaccine strain, SC602, were investigated in the Aotus model.
MATERIALS AND METHODS
Animal use and welfare.
Captive-bred Aotus nancymaae monkeys were purchased from the Facultad de Medicina Veterinaria de la Universidad Nacional Mayor de San Marcos, Lima, Peru, and shipped to U.S. Naval Medical Research Unit No. 6 (NAMRU-6) in Lima, Peru, for the study. The animals had not previously been used in a Shigella study. The study was conducted in an Association for the Assessment and Accreditation of Laboratory Animal Care, International, accredited vivarium with local approval by the NAMRU-6 Institutional Animal Care and Use Committee (IACUC), second-level approval from the U.S. Navy Bureau of Medicine and Surgery, and was approved by the Peruvian Dirección General Forestal y de Fauna Silvestre (resolution number 0023-2011-AG-DGFFS-DGEFFS). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for Care and Use of Laboratory Animals (16).
The animals were identified by unique tattoo numbers on their abdomens and were maintained in pairs when not required to be individually housed for sample collection. Prior to inclusion in the study, animals were screened by stool cultures for existing infection with Shigella spp. and for prior Shigella exposure by enzyme-linked immunosorbent assay (ELISA) for anti-S. flexneri 2a lipopolysaccharide (LPS) serum IgG titers. Those animals meeting the inclusion criteria (negative stool cultures and IgG titers of ≤20) were randomized to the various treatment groups. Aotus monkeys used in the challenge dose-finding study had a mean (± standard deviation [SD]) weight of 840 ± 66 g and a mean age of 19 ± 3 months on day 0 of the study. Aotus monkeys used in the vaccine immunogenicity and efficacy study had a mean weight of 868 ± 86 g and a mean age of 20 ± 5 months on day 0 of the study.
Preparation and administration of Shigella vaccine and challenge inoculums.
Shigella flexneri 2a strain 2457T is a wild-type Shigella strain that is Sereny test positive (17), pathogenic to monkeys (11, 18), and virulent in humans (19, 20). A vial of current good manufacturing practice (cGMP) S. flexneri 2a 2457T was reconstituted in saline, serially diluted, and plated for isolation on Trypticase soy agar (TSA) with 0.01% Congo red dye. After overnight incubation at 37°C, three small, smooth, Congo red-positive colonies were used to inoculate TSA plates (without Congo red) for confluent growth. The plates were harvested with 3.0 ml of cold phosphate-buffered saline (PBS), and the suspension was diluted based on a standardized OD600 (optical density at 600 nm) value. Shigella flexneri 2a vaccine strain SC602 has deletions in virG (icsA) and iuc (encoding aerobactin) genes (20). Strain SC602 is Congo red positive, indicating retention of the virulence plasmid, and unable to cause keratoconjunctivitis in the guinea pig eye (Sereny test negative) (21). S. flexneri 2a strain SC602 (20) was propagated using the same procedures used for S. flexneri 2a strain 2457T.
Monkeys were not allowed food overnight prior to administration of vaccine or challenge inoculums. Gastric acid production was inhibited with ranitidine (1.5 mg/kg of body weight) by intramuscular (i.m.) injection 90 min prior to inoculum delivery. Anesthetized animals (ketamine HCl; 50 mg/ml, 4 to 5 mg/kg, given i.m.) were orogastrically administered 5 ml of the rice-based buffer CeraVacx I (CeraProducts, Jessup, MD) using a single-use, sterilized, 5 Fr/Ch (1.7-mm) 16-in. (41-cm) feeding tube to neutralize the stomach contents. Immediately prior to inoculum delivery, the fluid content of the stomach was sampled and measured for pH. The challenge dose of S. flexneri 2a strain 2457T and the immunization dose of S. flexneri 2a strain SC602 were delivered orogastrically in a 5-ml volume using a new feeding tube.
Challenge dose-finding study design.
Groups (9 animals/group) of A. nancymaae were orogastrically inoculated with increasing doses (5 × 109, 5 × 1010, or 5 × 1011 CFU) of Shigella flexneri 2a 2457T. A fourth group of 10 animals was administered PBS. Nine weeks following primary challenge (day 63), all animals were rechallenged with 1 × 1011 CFU of S. flexneri 2a 2457T. Animals were observed for 10 days following each inoculation for symptoms of illness (described below) and then treated with enrofloxacin (5 mg/kg, i.m.) daily for 5 days.
Vaccine immunogenicity and efficacy study design.
Groups of eight A. nancymaae were orogastrically immunized on study days 0, 14, and 42 with 1 × 1010 or 1 × 1011 CFU of the live-attenuated vaccine strain Shigella flexneri 2a strain SC602. Another group was immunized with a subclinical dose (1 × 109 CFU) of S. flexneri 2a strain 2457T. A control group (10 Aotus monkeys) was inoculated with PBS on the same schedule. On study day 70, all animals were orogastrically challenged with 1 × 1011 CFU of S. flexneri 2a 2457T as described above.
Observations after vaccination and challenge.
Animals were observed for signs and symptoms of diarrhea twice daily prior to each vaccination or challenge, for 5 days after vaccination, and for 10 days after challenge. Observations included activity level, stool consistency, and the presence of blood observed in the feces. Activity level was scored on a scale of 0 to 3 as follows: 0, active and responsive; 1, reduced activity; 2, immobile; 3, recumbent. Fecal occult blood was determined by a hemoccult test (Hemoccult II SENSA; Beckman Coulter, Fullerton, CA) according to the manufacturer's instructions. Stools were graded daily as follows: grade 1 (formed, firm stool pellets), grade 2 (formed but soft stool pellets or droppings), grade 3 (loose, unformed feces), grade 4 (watery, nonclear feces), and grade 5 (watery, clear liquid stools). Stools graded 1 or 2 were considered normal, whereas stools graded 3, 4, or 5 were considered abnormal. The case definition of a diarrhea episode was defined as the passing of a grade 3 or higher stools for at least two consecutive days during the observation period. The duration of diarrhea was defined as the time between the first day of a diarrhea episode and the last day of diarrhea preceding two consecutive diarrhea-free days. Animals meeting the case definition of diarrhea prior to challenge were excluded from data analysis. Clinical symptoms of Shigella-induced gastroenteritis were defined as evidence of Shigella colonization (PCR or isolation) and either (i) an episode of diarrhea (as defined above), (ii) blood in the stool (occult, gross, or melena) for two consecutive days, or (iii) death.
Clinical sample collection and processing.
Blood samples were collected, and serum samples were stored at −80°C until assayed by ELISA. Blood samples were collected from individual animals on study days 0, 7, 14, 21, 49, 70, and 77 in the challenge dose-finding study. In the vaccine immunogenicity and efficacy study, blood samples were collected on study days 0, 21, 49, 70, 77, and 84. Stool samples were collected from cage drop pans before immunization or challenge and daily for 10 days after each vaccination or challenge.
Shigella colonization determination.
Colonization of Aotus after vaccination or challenge was determined as previously described for rhesus macaques (22). Briefly, stool was streaked onto Hektoen enteric agar plates. Suspected Shigella colonies were confirmed by slide agglutination with commercially available S. flexneri 2a antiserum (Denka Seiken Co.) or by colony immunoblotting with the anti-IpaB monoclonal antibody 2F1 (23). Stool samples testing negative for Shigella were subjected to PCR analysis targeting the ipaH gene (22).
Immunogenicity assessment.
Serum antigen-specific antibody responses were assessed by an ELISA as previously described (24) with the following modifications: the antigen coating concentrations were 10 μg/ml for S. flexneri 2a LPS and 1 μg/ml for S. flexneri 2a Invaplex (25), and purified IpaB and IpaC were used in a total assay volume of 100 μl. Conjugated rabbit anti-Aotus IgG and anti-Aotus IgA secondary antibodies (Lampire Biological Labs Inc., Pipersville, PA) were used to detected antigen-bound serum antibodies. Seroconversion was defined as ≥4-fold increase in the titer over the baseline level.
Statistical analysis.
Intragroup comparisons of clinical and immunologic outcomes were performed using nonparametric tests for continuous outcomes (Wilcoxon rank sum test for comparing the values for two groups; Kruskal-Wallis test for comparing the values for more than two groups) and Fisher's exact tests for nominal outcomes. All statistical tests were interpreted in a two-tailed fashion with acceptance of significance set at the P < 0.05 level.
RESULTS
Dose-finding and rechallenge study. (i) Clinical symptoms, microbiology, and challenge results.
Results from a preliminary, pilot study indicated that oral challenge with 5 × 109 CFU of S. flexneri 2a strain 2457T induced diarrhea in 1 of 3 animals (33%) and did not cause significant disease in the remaining animals (data not shown). Therefore, three groups of A. nancymaae monkeys were orally challenged with S. flexneri 2a 2457T at either 5 × 109, 5 × 1010, or 5 × 1011 CFU (Table 1) to determine a challenge dose that induced diarrhea in at least 75% of the animals. Aotus monkeys in the control group were mock challenged with PBS. One of the 10 animals (10%) in the PBS control group was positive for diarrhea for 9 days after inoculation with PBS. Shigella spp. were not recovered from the animal in fecal cultures, and the stools were ipaH negative by PCR at all time points.
TABLE 1.
Incidence of diarrhea and clinical symptoms after oral challenge of Aotus nancymaae with escalating doses of S. flexneri 2a strain 2457T and homologous rechallengea
| Group | Treatment (dose [CFU]) | Primary challenge |
Homologous rechallenge |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of animals | Incidence (%) of diarrheab | Incidence (%) of clinical symptomsc | P valued | No. of animals | Incidence (%) of diarrhea | Incidence (%) of clinical symptoms | P value | ||
| 1 | S. flexneri 2a 2457T (5 × 109) | 8e | 25 | 50 | 0.558 | 8e | 25 | 38 | 0.145 |
| 2 | S. flexneri 2a 2457T (5 × 1010) | 9 | 56 | 56 | 0.057 | 7e,f | 43 | 71 | 1.000 |
| 3 | S. flexneri 2a 2457T (5 × 1011) | 9 | 100 | 100 | 0.0001 | 8f | 38 | 50 | 0.321 |
| 4 | PBS | 10 | 10 | 10 | 10 | 50 | 80 | ||
The groups of Aotus nancymaae monkeys were challenged with S. flexneri 2a strain 2457T, treated with 1 × 1011 CFU of S. flexneri 2a 2457T, and then rechallenged with a homologous strain.
Diarrhea was defined as at least one loose-watery stool on at least two consecutive days during the observation period (10 days).
Clinical symptoms of Shigella-induced gastroenteritis were defined as evidence of Shigella colonization (PCR or isolation) and either (i) an episode of diarrhea, (ii) blood in the stool (occult, gross, or melena) for two consecutive days, or (iii) death.
The P values were calculated by Fisher's exact test compared to control group inoculated with PBS.
One animal excluded from data analysis due to diarrhea for 2 days prior to challenge.
One animal euthanized after the primary challenge.
In contrast, S. flexneri 2a strain 2457T induced diarrhea in 25, 56, and 100% of animals orally inoculated with 5 × 109, 5 × 1010, and 5 × 1011 CFU, respectively. Disease was characterized as loose, low-volume stools with either gross or occult blood present. There was no significant difference in the colonization rate or duration between dose groups. Only the group administered 5 × 1011 CFU of S. flexneri 2a 2457T had a significantly higher number of days of diarrhea (Table 2) compared to PBS controls. On day 2 after challenge, one animal inoculated with 5 × 1011 CFU was euthanized due to severe disease. An additional animal that was inoculated with 5 × 1010 CFU was euthanized 6 days postchallenge due to bloody vomitus and lethargy. Necropsy of the animals revealed hemorrhagic and necrotic small intestines and stomachs. Tissue samples collected from the colon, ileum, and stomach were macerated, cultured, and tested positive for S. flexneri. Tissue samples from the peritoneum tested negative for enteropathogens.
TABLE 2.
Diarrhea and colonization after oral challenge of A. nancymaae with S. flexneri 2a strain 2457T
| Treatment (CFU) | No. of animals | Diarrheaa |
Colonizationb |
||||
|---|---|---|---|---|---|---|---|
| No. of cases | Mean no. of days to onset (range) | Mean no. of days to illness (range) | % Incidence | Median no. of days to onset | Median no. of days of duration (range) | ||
| S. flexneri 2a 2457T (5 × 109) | 8c | 2 | 4 (3–5) | 5 (2–8) | 100 | 1 | 10 (3–10) |
| S. flexneri 2a 2457T (5 × 1010) | 9 | 5 | 2 (1–4) | 7 (5–10)d | 100 | 1 | 7.5 (2–10) |
| S. flexneri 2a 2457T (5 × 1011) | 9 | 8e | 3 (1–3) | 5 (2–10)f | 100 | 1 | 8.5 (5–10) |
| PBS | 10 | 1 | 1 | 0.9 (0–9) | 0 | ||
Diarrhea defined as at least one loose-watery stool on at least two consecutive days during the observation period (10 days) postchallenge.
Colonization assessed by plating on HE agar with confirmatory slide agglutination or colony blotting. Negative samples confirmed with ipaH-specific PCR on frozen stool specimens.
One animal excluded from data analysis due to diarrhea for 2 days prior to challenge.
One animal removed from the study on day 6 postchallenge; full duration data not collected.
One animal removed from the study at 2 days postchallenge; excluded from diarrhea incidence data analysis.
P = 0.002 (Mann-Whitney; two-tailed, α = 0.05) compared to PBS control group.
The incidence of clinical symptoms, which captures disease and death due to infection, was used to calculate a dose of 1 × 1011 CFU to result in a 75% attack rate. The dose of S. flexneri 2a strain 2457T expected to induce the targeted 75% attack rate in naive Aotus nancymaae was calculated by applying a linear fit to the line generated after plotting the log10-transformed doses (CFU) of S. flexneri 2a 2457T versus the attack rate achieved at each dose and interpolating the expected dose.
The animals were allowed to rest for 9 weeks, and then all groups were orally challenged with 1 × 1011 CFU of S. flexneri 2a strain 2457T. Veterans from the primary challenge were used to determine whether protection could be achieved after homologous rechallenge, whereas the veteran PBS control group was used to confirm that the calculated dose would result in a ≥75% attack rate.
Challenge of the veteran PBS groups with S. flexneri 2a strain 2457T resulted in an 80% attack rate confirming previous results (Table 1). Animals previously infected with S. flexneri 2a 2457T were not protected upon subsequent rechallenge with a homologous strain (Table 1) with the incidence of clinical symptoms ranging from 38% to 71%. There was no significant difference in the duration of diarrhea or colonization among the study groups. Three animals were euthanized due to complications from the second challenge, two animals from the group that previously received PBS during the primary challenge phase of the experiment and one animal that had received 5 × 1011 CFU. All euthanized animals had occult blood in the stool and bloody vomitus prior to death. Upon necropsy, blood and colitis were noted in the distal colon of two animals (Shigella veteran and PBS control veteran) with no pathology in the stomach or small intestine. Necropsy of the third animal (PBS control veteran) revealed loose stool without blood in the large intestine and necrosis in the stomach.
(ii) Immunological assessment.
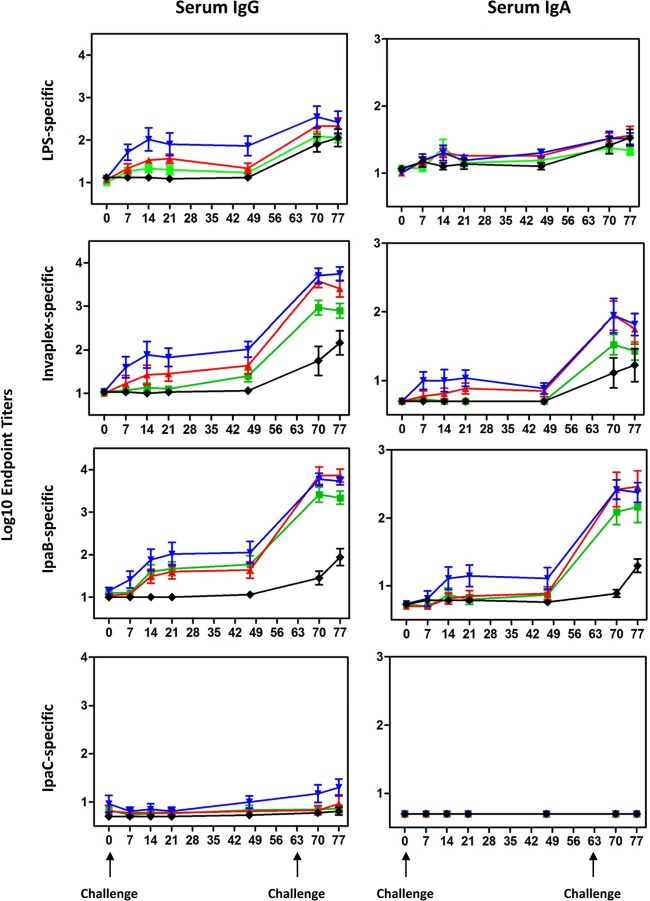
Individual animals were bled before challenge on day 0 and on days 7, 14, 21, and 49 after the primary challenge. Blood was also collected 1 and 2 weeks (days 70 and 77) after the second challenge. Serum IgG and IgA endpoint titers directed against S. flexneri 2a LPS and S. flexneri 2a Invaplex, IpaB, and IpaC were determined by ELISA (Fig. 1). Shigella-specific antibodies were not detected in serum of animals treated with PBS at any time point during the primary challenge phase of the study. Furthermore, baseline Shigella-specific antibodies were low across all groups prior to challenge. The serum IgG titers directed against LPS, Invaplex, IpaB, and IpaC followed a dose-dependent relationship with animals receiving the highest dose of S. flexneri 2a strain 2457T possessing the highest antigen-specific antibody titers (Fig. 1). Seroconversion to Invaplex (which includes LPS, IpaB, and IpaC antigens [25]) was challenge dose dependent with 25%, 63%, and 88% of Aotus monkeys challenged with 5 × 109, 5 × 1010, and 5 × 1011 CFU, respectively, having at least a 4-fold increase in serum IgG titers. IpaC-specific titers were low to undetectable across all groups after the primary challenge. A slight decline was noted in the LPS- and Invaplex-specific IgG titers between samples collected on day 21 and day 49, whereas IpaB and IpaC titers remained constant or increased slightly during the same time period. Shigella-specific serum IgG titers increased ∼0.5 to 2 log units after rechallenge with a homologous serotype. The kinetics of the serum IgG response directed against the Shigella antigens during the second challenge phase of the study in the PBS control group largely mirrored the kinetics of the response previously demonstrated after challenge with 5 × 1011 CFU in the primary challenge phase of the experiment.
FIG 1.
Kinetics of the Shigella-specific serum IgG and IgA responses in A. nancymaae after oral challenge with escalating doses of S. flexneri 2a strain 2457T and rechallenge with 1 × 1011 CFU of the homologous strain. Groups of A. nancymaae monkeys were orally challenged on study day 0 with 5 × 109 (green square), 5 × 1010 (red triangle), or 5 × 1011 (blue inverted triangle) CFU of S. flexneri 2a 2457T. Another group was mock challenged with PBS (black diamond). On study day 63, all groups were orally challenged with 1 × 1011 CFU of S. flexneri 2a 2457T. Group mean titers ± 1 standard deviation (error bars) are plotted. The days the animals were challenged are indicated by the black arrows under the graphs at the bottom of the figure.
Shigella-specific serum IgA responses after primary challenge with 5 × 109 and 5 × 1010 CFU were low, with less than 25% of animals seroconverting to any of the antigens (Fig. 1). Moderate levels of Shigella-specific IgA were elicited after oral inoculation with 5 × 1011 CFU of S. flexneri 2a strain 2457T with 38 to 50% of Aotus seroconverting after primary challenge. After rechallenge, there were significant increases in Invaplex- and IpaB-specific serum IgA titers, whereas IgA responses directed against IpaC and LPS were largely unchanged (Fig. 1).
Reactogenicity and colonization after oral immunization of A. nancymaae with strain SC602 or wild-type S. flexneri 2a strain 2457T.
The reactogenicity, immunogenicity, and protective efficacy of a live-attenuated Shigella flexneri 2a vaccine strain, SC602, was assessed in the Aotus nancymaae model. SC602 has been previously shown to be immunogenic and protective against shigellosis in clinical trials (20) and in the rhesus macaque model (M. M. Venkatesan, unpublished data). In addition, a group of Aotus monkeys were immunized with S. flexneri 2a 2457T (1 × 109 CFU) to test the hypothesis that multiple immunizations with a subclinical dose could induce a protective immune response. As presented above, primary challenge with S. flexneri 2a 2457T at 5 × 109 CFU followed by rechallenge with 1 × 1011 CFU resulted in a strong immune response directed against multiple Shigella antigens (Fig. 1) conveying partial protection as evidenced by a low rate of diarrhea (Table 2), suggesting that low-level infections could confer protection against a larger bolus of Shigella.
Groups were orally immunized on days 0, 14, and 42 with strain SC602 (1010 or 1011 CFU/dose) or S. flexneri 2a strain 2457T (109 CFU/dose). Clinical symptoms and bacterial colonization were monitored for 10 days after each immunization (Table 3). As expected, animals mock immunized with PBS were not colonized with shigellae. All animals immunized with SC602 were colonized after each oral immunization for 1 to 8 days. The number of diarrhea cases was also low after each immunization with SC602. In contrast, oral immunization with S. flexneri 2a 2457T induced diarrhea in 75% (6/8) of animals after each immunization, which was significantly higher than the number of cases of diarrhea in PBS controls (P = 0.007 by Fisher's exact test), and colonization rates were also substantial (75 to 100%).
TABLE 3.
Clinical symptoms after oral immunization with either live-attenuated vaccine strain SC602 or wild-type S. flexneri 2a strain 2457T
| Time and clinical parameter | Value for treatment groupa |
|||
|---|---|---|---|---|
| SC602 (n = 8) (1 × 1010 CFU) | SC602 (n = 8) (1 × 1011 CFU) | S. flexneri 2a 2457T (n = 8) (1 × 109 CFU) | PBS (n = 10) | |
| After vaccination 1 | ||||
| No. of cases of diarrheab | 1 | 1 | 6 | 2 |
| Mean day of onset (range) | 7 (NA) | 1 (NA) | 3.8 (1–8) | 7 (1–9) |
| Mean no. of days of illness (range) | 2 (NA) | 10 (NA) | 3.5 (2–9) | 3.5 (2–5) |
| % Colonizationc | 100 | 100 | 75 | 0 |
| Median duration (range) | 1 (1–3) | 4 (1–8) | 7.5 (2–10) | |
| After vaccination 2 | ||||
| No. of cases of diarrhea | 2 | 1 | 6 | 0 |
| Mean day of onset (range) | 2.5 (2–3) | 1 (NA) | 2 (1–5) | 0 |
| Mean no. of days of illness (range) | 3 (2–4) | 5 (NA) | 6 (2–10) | 0 |
| % Colonization | 100 | 100 | 100 | 0 |
| Median duration (range) | 2 (1–3) | 2 (2–4) | 5 (1–8) | |
| After vaccination 3 | ||||
| No. of cases of diarrhea | 0 | 2 | 6 | 0 |
| Mean day of onset (range) | 0 (NA) | 1 (NA) | 2.8 (1–6) | 0 |
| Mean no. of days of illness (range) | 0 (NA) | 8 (6–10) | 4 (2–8) | 0 |
| % Colonization | 100 | 100 | 100 | 0 |
| Median duration (range) | 2 (1–2) | 2 (1–3) | 6 (1–8) | |
NA, not applicable.
Diarrhea defined as at least one loose-watery stool on at least two consecutive days during the observation period (10 days).
Colonization assessed by plating of HE agar with confirmatory slide agglutination or colony blotting. Negative samples confirmed with ipaH-specific PCR on frozen stool specimens.
Protective efficacy after oral immunization of Aotus with live-attenuated strain SC602 or wild-type S. flexneri 2a strain 2457T.
Animals orally immunized on days 0, 14, and 42 were subsequently challenged with an oral dose of S. flexneri 2a strain 2457T (1 × 1011 CFU) on day 70 and monitored for 10 days (Table 4). Two animals immunized with strain SC602 were excluded from analysis due to the onset of diarrhea prior to challenge. The diarrhea attack rate in the placebo group was 70% (7/10 animals) and 14% (1/7 animals) in groups immunized with 1 × 1010 or 1 × 1011 CFU of strain SC602 (80% protective efficacy; P = 0.05). Comparison of the PBS control group to both groups immunized with SC602 (1 × 1010 and 1011 CFU) resulted in 80% protective efficacy (P = 0.01 by Fisher's exact test). Immunization with S. flexneri 2a 2457T did not result in significant protection (46%; P = 0.34), a delay in the mean day of onset or a decrease in the duration of illness (Table 5). There was a significant reduction in the duration of diarrhea after challenge in groups orally immunized with 1 × 1010 CFU of SC602 (P = 0.05 by Mann-Whitney test) and 1 × 1011 CFU of SC602 (P = 0.025) compared to the PBS control group. There was no difference in the colonization rate or duration after oral challenge with S. flexneri 2a 2457T in any of the immunized groups and the placebo controls.
TABLE 4.
Diarrhea incidence, clinical symptoms, and protective efficacy in Aotus nancymaae orally immunized with live-attenuated SC602 vaccine strain or S. flexneri 2a strain 2457T and then challenged with S. flexneri 2a 2457T
| Treatment (CFU dose) | No. of animals | Diarrhea incidence (%)a | Clinical symptoms (%)b | Protective efficacy (%)c | P valued |
|---|---|---|---|---|---|
| SC602 (1 × 1010) | 7e | 14 | 14 | 80 | 0.05 |
| SC602 (1 × 1011) | 7e | 14 | 14 | 80 | 0.05 |
| S. flexneri 2a 2457T (1 × 109) | 8 | 38 | 38 | 46 | 0.34 |
| PBS | 10 | 70 | 70 |
Diarrhea defined as at least one loose-watery stool on at least two consecutive days during the observation period (10 days).
Clinical symptom defined as diarrhea, bloody stools for 2 days, or death.
Protective efficacy = [(percentage of clinical symptoms of the control group − percentage of clinical symptoms in the vaccinated group)/(percentage of clinical symptoms of the control group)].
Fisher's exact test compared to the PBS control group.
One animal excluded from data analysis due to diarrhea for 2 days prior to challenge.
TABLE 5.
Diarrhea duration, clinical symptoms, and colonization after oral immunization of A. nancymaae with live-attenuated vaccine strain SC602 or S. flexneri 2a strain 2457T and oral challenge with S. flexneri 2a 2457Ta
| Treatment | No. of animals | Diarrheab |
Colonizationc |
||||
|---|---|---|---|---|---|---|---|
| No. of cases | Mean no. of days to onset (range) | Mean no. of days illness (range) | % Incidence | Median no. of days to onset | Median no. of days duration (range) | ||
| SC602 (1 × 1010 CFU) | 7d | 1 | 3 | 4 | 100 | 1 | 7.9 (3–10) |
| SC602 (1 × 1011 CFU) | 7d | 1 | 2 | 2 | 100 | 1 | 8.4 (4–10) |
| S. flexneri 2a 2457T (1 × 109 CFU) | 8 | 3 | 5 (3–6) | 3 (2–5) | 100 | 1 | 6.6 (2–10) |
| PBS | 10 | 7 | 3.3 (1–7) | 4.6 (2–8) | 100 | 1 | 8.0 (4–10) |
Groups of monkeys were orally immunized on days 0, 14, and 42 with either live-attenuated Shigella vaccine strain SC602 or with wild-type S. flexneri 2a 2457T at a subclinical dose. The animals were then orally challenged with 1 × 1011 CFU of S. flexneri 2a 2457T on day 70.
Diarrhea defined as at least one loose-watery stool on at least two consecutive days during the observation period (10 days).
Colonization assessed by plating of HE agar with confirmatory slide agglutination or colony blotting. Negative samples confirmed with IpaH-specific PCR on frozen stool specimens.
One animal excluded from data analysis due to diarrhea prior to the challenge.
Immune responses after oral immunization of Aotus with live-attenuated strain SC602 or wild-type S. flexneri 2a strain 2457T and subsequent oral challenge.
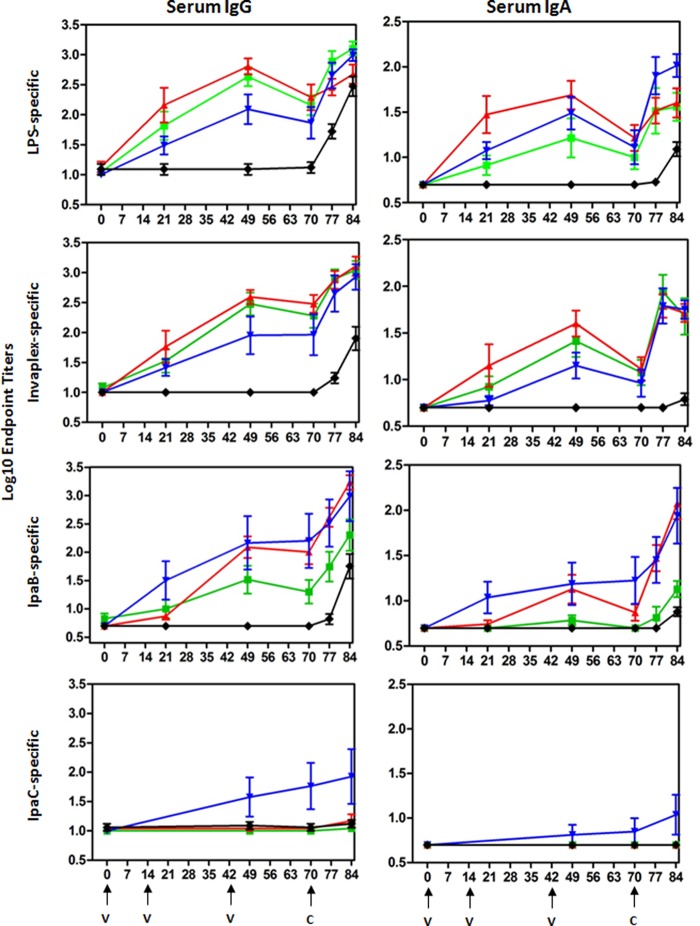
Blood samples collected on study days 0, 21, 49, 70, 77, and 84 were assayed by ELISA for IgG and IgA endpoint titers directed against S. flexneri 2a LPS, Invaplex, IpaB, and IpaC (Fig. 2). Animals mock immunized with PBS did not mount an antigen-specific serum IgG or IgA response during the immunization phase of the study. Furthermore, Shigella antigen-specific serum IgG and IgA titers on study day 0 were low in all experimental groups. In contrast, robust levels of serum IgG directed against S. flexneri 2a LPS, Invaplex, and IpaB were induced after three oral immunizations with strain SC602 or S. flexneri 2a strain 2457T, and the responses were of significantly higher magnitude (P < 0.01) than the responses in the PBS control group. Seroconversion after immunization with strain SC602 was dose dependent and most evident in antigen-specific serum IgA. For example, 43% (3/7) of Aotus monkeys receiving SC602 (1 × 1010 CFU) seroconverted to LPS compared to 100% (7/7) of animals receiving SC602 (1 × 1011 CFU). Similarly, none of the animals immunized with SC602 (1 × 1010 CFU) had IpaB-specific serum IgA, whereas 57% (4/7) of Aotus immunized with SC602 (1 × 1011 CFU) seroconverted to IpaB. Interestingly, only animals immunized with S. flexneri 2a 2457T had detectable anti-IpaC serum IgG (38% [3/8]) and IgA (13% [1/8]) responses after immunization, albeit at low levels. However, there was no significant difference in seroconversion rates between animals immunized with SC602 (1 × 1010 or 1 × 1011 CFU) and animals immunized with S. flexneri 2a 2457T across all antigens assayed. An increase in serum IgG and IgA directed against LPS, Invaplex, and IpaB was demonstrated after challenge, indicating that vaccination effectively primed the ensuing immune response.
FIG 2.
Kinetics of the Shigella-specific serum IgG and IgA responses in A. nancymaae after oral immunization with live-attenuated vaccine strain SC602 or wild-type S. flexneri 2a strain 2457T and oral challenge with S. flexneri 2a 2457T. Groups of A. nancymaae monkeys were orally immunized on study days 0, 14, and 42 with 1 × 1010 CFU of SC602 (green square), 1 × 1011 CFU of SC602 (red triangle), or 1 × 109 (blue inverted triangle) CFU of S. flexneri 2a 2457T (black diamond). The control group was mock immunized with PBS (black). On study day 70, all groups were orally challenged with 1 × 1011 CFU of S. flexneri 2a 2457T. Group mean titers ± 1 standard deviation are plotted. The days of immunization (v) and the days of challenge (c) are indicated by the black arrows under the graphs at the bottom of the figure.
DISCUSSION
The three most common bacterial pathogens responsible for traveler's diarrhea include ETEC, Campylobacter, and Shigella (6). In addition, significant morbidity and mortality are associated with these enteric pathogens in impoverished areas where the disease is endemic (26). Substantial efforts over the past decade have resulted in the generation of several vaccine candidates to prevent the causes of diarrhea by these enteric bacterial pathogens. Ideally, a combination vaccine capable of protecting against ETEC, Shigella, and Campylobacter will be developed and deployed. A single animal model to evaluate immunogenicity and efficacy of a combination enteric vaccine may greatly facilitate development and evaluation.
The A. nancymaae model has been used to evaluate the immunogenicity and efficacy of several ETEC (15) and Campylobacter (14, 27) vaccines. The attack rates in naive Aotus monkeys orally inoculated with 5 × 1011 to 7 × 1011 CFU of C. jejuni are typically ≥70%. Similar attack rates are achieved after oral inoculation of naive Aotus with 1 × 1011 to 5 × 1011 CFU of ETEC. Disease in the ETEC and Campylobacter challenge models is typically characterized by diarrhea and bacterial colonization evidenced by positive stool culture. Although there are similarities between the three challenge models in terms of infectious dose and inducing diarrhea, there are also several key differences between the ETEC- and Campylobacter-Aotus models compared to the Shigella model. In the Shigella model, melena or black tarry stool with gross blood is a typical outcome, whereas gross blood is rarely seen in the ETEC and Campylobacter models.
Another characteristic of the Shigella-Aotus challenge model that differs from the ETEC- and Campylobacter-Aotus challenge models is death in a subset of animals. Necropsies of Aotus monkeys challenged with Shigella revealed hemorrhagic and necrotic small intestines and stomachs in a subset of animals, similar to reports using rhesus macaques (10). Death after oral challenge of nonhuman primates with shigellae has been documented in several studies (28, 29). Oral challenge of M. fascicularis monkeys with 1 × 1010 or 1 × 1011 CFU of S. flexneri 2a strain 2457T resulted either in death 3 and 4 days postinoculation or a moribund state requiring humane euthanasia (28, 29). After oral challenge of 40 rhesus monkeys with S. flexneri 2a 2457T (3.2 × 1010 CFU), five animals died, reportedly due to necrotizing enteritis characteristic of acute shigellosis (29). In the Aotus model, several animals were humanely euthanized or died within a week of oral challenge with ≥5 × 1010 CFU of S. flexneri 2a 2457T. In the subsequent study in which Aotus monkeys were challenged with 1 × 1011 CFU of S. flexneri 2a 2457T, none of the animals died or required humane euthanasia. It is difficult to speculate on the disparate results between the two Aotus studies due in part to the small number of animals in each study. Future work using more animals should help to address the inconsistency.
The majority of results achieved in the current study are consistent with reports describing oral infection of rhesus monkeys with virulent shigellae. The similarities between the two animal models include the dose (∼1 × 1011 CFU) required for reproducible infection (28–30), time course and severity of the disease (28, 30, 31), and protection against infection after immunization of rhesus monkeys with live-attenuated SC602 vaccine strain (unpublished results). One difference in the results achieved in the Aotus model compared to the rhesus monkey model is protection afforded after homologous rechallenge. A seminal study conducted by Formal and colleagues clearly demonstrated that prior infection with S. flexneri 2a protected rhesus monkeys against subsequent challenge with homologous S. flexneri 2a but not against heterologous Shigella sonnei, despite serum IgG responses directed against the highly conserved Ipa proteins (32). In the rhesus monkey model, serotype-specific LPS responses were suggested as the protective antigen. In a similar fashion, Aotus monkeys were challenged with increasing doses of S. flexneri 2a, rested for 9 weeks, and then rechallenged with S. flexneri 2a. In contrast to rhesus monkeys, no protection was afforded after a homologous back challenge of the Aotus monkeys. The discordant results between the two studies may reflect differences in genetic background and susceptibility to infection but may also be a product of significant differences in the experimental procedures. In the Aotus studies, all monkeys were screened for serum IgG responses to S. flexneri 2a LPS to ensure that there was no preexisting immunity, whereas Formal et al. focused on stool cultures to ensure that no carrier state or active infection was identified. The inoculum dose used by Formal et al. was 2 × 1010 CFU delivered orally in brain heart infusion (BHI) to animals weighing 2.3 to 3.2 kg. The Aotus monkeys in the present study weighed ∼850 g and were inoculated with ∼1 × 1011 CFU delivered in a rice-based buffer. The challenge inoculum/weight ratio in the rhesus monkey study (2 × 1010 CFU/2 to 3 kg) may have resulted in a less robust challenge (54% attack rate). Finally, the BHI nutrient medium used in the challenge bolus given in the Formal et al. study may have also impacted gene expression and perhaps invasiveness of the shigellae.
In the challenge/rechallenge study, serum IgG responses directed against S. flexneri 2a LPS were low after the primary infection with 5 × 109 or 5 × 1010 CFU and of moderate magnitude after challenge with 5 × 1011 CFU. However, upon rechallenge, all groups demonstrated a significant boost in the anti-LPS serum IgG titers. These results suggested that perhaps a single infection of Aotus monkeys with S. flexneri 2a did not sufficiently prime the immune system to provide protective efficacy, but perhaps repeated infections may be required to produce the necessary protective immune response.
To test this hypothesis, Aotus monkeys were immunized with a subclinical dose of S. flexneri 2a (5 × 109 CFU) on days 0, 14, and 42. Anti-LPS, anti-IpaB, and anti-IpaC serum IgG and IgA titers were similar in groups administered S. flexneri 2a twice (challenge/rechallenge study) and groups administered S. flexneri 2a three times. In agreement with previous results, Aotus monkeys administered S. flexneri 2a strain 2457T were not significantly protected against back challenge with a homologous Shigella serotype. However, in the same study, Aotus monkeys immunized with strain SC602 (1010 or 1011 CFU) were protected against challenge with S. flexneri 2a strain 2457T. There were no significant differences in serum IgG or IgA responses specific for Invaplex, LPS, and IpaB in groups immunized with SC602 (1010 or 1011CFU) and groups immunized with S. flexneri 2a 2457T. Mucosal immune responses were not assessed in the current study and may be responsible for the differences in protective efficacy obtained between 2457T and SC602. After inoculation with 2457T, 75% of Aotus monkeys had episodes of diarrhea, whereas only 11 to 22% of Aotus monkeys immunized with SC602 experienced similar loose stools. Episodes of diarrhea did not result in a significant reduction in colonization but may have impacted the generation of robust anti-LPS serum antibodies or affected the induction of gut-homing mucosal IgA in the large intestine if an adequate microenvironment was not maintained.
Several other nonhuman primate models (other than Aotus nancymaae) have been utilized to investigate Shigella pathogenesis and the immunogenicity and efficacy of Shigella vaccines, including both rhesus monkeys (Macaca mulatta) and cynomolgus monkeys (Macaca fascicularis). Shigella flexneri (1 × 1011 CFU) fed to rhesus monkeys resulted in lesions in the colonic epithelium (33). Rhesus monkeys fed 108 to 1010 CFU of S. flexneri 2a had clinical signs of acute shigellosis within 48 h of challenge (34), including lethargy, prostration, and diarrhea with liquid or semisolid mucohemorrhagic stools (12). In addition to rhesus monkeys, cynomolgus monkeys have been infected with Shigella spp. (35). Interestingly, intragastric (1011 CFU) but not intraduodenal (109 CFU) injection of Shigella dysenteriae 1 was able to induce shigellosis despite colonization and serum antibody responses after intraduodenal administration.
Shigella flexneri 2a vaccine strain SC602 carries deletions in virG (icsA) and iuc (encoding aerobactin) genes (21). SC602 is Congo red positive, indicating that it has retained the virulence plasmid and is unable to cause keratoconjunctivitis in the guinea pig eye (Sereny test negative) (21). In the rhesus model, the monkeys were orally immunized on days 0, 10, and 20 with 8 × 1010 CFU of strain SC602. The immunized rhesus monkeys (≥44%) secreted liquid stools with mucus within 72 h after each vaccination with SC602 (unpublished results). After the first vaccination, all monkeys shed S. flexneri 2a for 3 days. All immunized animals also shed S. flexneri 2a after the second and third vaccinations, but the carriage rate was diminished with each successive immunization. On study day 48, control animals (n = 8) and SC602-immunized animals (n = 16) were orogastrically challenged with 1 × 1011 CFU of S. flexneri 2a strain 2457T. Vaccination of rhesus monkeys with strain SC602 was associated with 75% protection against overt dysentery (P = 0.002). Similar to the rhesus model, 80% protection was achieved in the current study after oral immunization of Aotus nancymaae with SC602 on days 0, 14, and 42.
Oral immunization of humans with ≥106 CFU of strain SC602 causes shigellosis in the majority of volunteers (20). In contrast, immunization with 104 CFU results in transient fever or mild diarrhea in a small percentage of volunteers. Moreover, volunteers immunized with strain SC602 (104 CFU) and subsequently challenged with wild-type S. flexneri 2a strain 2457T were completely protected against fever and severe shigellosis (4), while six of seven controls experienced shigellosis. Expanded safety evaluation of strain SC602 (104 CFU) resulted in fever and diarrhea in 15% of volunteers, as well as headaches (35%) and abdominal cramps (24%) warranting further attenuation for clinical development (36). Similar levels of loose stools were induced after administration of SC602 to rhesus macaques and Aotus nancymaae, albeit at higher dose levels, suggesting that the monkey models may mimic, in part, the immunogenicity and reactogenicity in humans.
The described Aotus nancymaae model provides another means to study pathogenesis and Shigella vaccine immunogenicity and efficacy. Moreover, the model opens the possibility for future testing of combination vaccines to combat infection with the three most prevalent enteric bacterial pathogens encountered by travelers, military, and most importantly, children living in areas where these pathogens are endemic. Significant research needs to focus on building upon immunological evaluation in the Aotus model to include assessing antigen-specific fecal IgA, memory B cells, and IgA-secreting plasma cells in search of immune correlates of protection. Future efforts will also focus on expanding the challenge model to include additional Shigella serotypes to facilitate the efficacy testing of different Shigella vaccine formulations and constructs and exploring the potential of a broad-based immune response capable of cross-protecting against multiple, relevant Shigella serotypes.
ACKNOWLEDGMENTS
We are grateful to K. Ross Turbyfill for providing purified proteins, antibodies, and Shigella LPS and to Kristen Clarkson and Gladys Nunez for excellent technical assistance.
This work was funded by the Military Infectious Disease Research Program (MIDRP).
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. government. The mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. The funding agency did not play a role in the study design, collection, analysis, or interpretation of data, in writing the report, or in the decision to submit the article for publication.
Footnotes
Published ahead of print 4 March 2014
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77:651–666 [PMC free article] [PubMed] [Google Scholar]
- 3.Garrett V, Bornschlegel K, Lange D, Reddy V, Kornstein L, Kornblum J, Agasan A, Hoekstra M, Layton M, Sobel J. 2006. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmission. Epidemiol. Infect. 134:1231–1236. 10.1017/S0950268806006182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan O, Crook P, Cheasty T, Jiggle B, Giraudon I, Hughes H, Jones SM. 2006. Shigella sonnei outbreak among homosexual men, London. Emerg. Infect. Dis. 12:1458–1460. 10.3201/eid1209.060282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SF, Riddle MS, Wierzba TF, Messih IA, Monteville MR, Sanders JW, Klena JD. 2006. Epidemiology and genetic characterization of Shigella flexneri strains isolated from three paediatric populations in Egypt (2000-2004). Epidemiol. Infect. 134:1237–1248. 10.1017/S095026880600642X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle MS, Sanders JW, Putnam SD, Tribble DR. 2006. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am. J. Trop. Med. Hyg. 74:891–900 [PubMed] [Google Scholar]
- 7.Mallett CP, VanDeVerg L, Collins HH, Hale TL. 1993. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine 11:190–196. 10.1016/0264-410X(93)90016-Q [DOI] [PubMed] [Google Scholar]
- 8.Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. 1991. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect. Immun. 59:4075–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim DH, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. 2007. New animal model of shigellosis in the guinea pig: its usefulness for protective efficacy studies. J. Immunol. 178:2476–2482 [DOI] [PubMed] [Google Scholar]
- 10.Kent TH, Formal SB, LaBrec EH, Sprinz H, Maenza RM. 1967. Gastric shigellosis in rhesus monkeys. Am. J. Pathol. 51:259–267 [PMC free article] [PubMed] [Google Scholar]
- 11.Formal SB, Hale TL, Kapfer C, Cogan JP, Snoy PJ, Chung R, Wingfield ME, Elisberg BL, Baron LS. 1984. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect. Immun. 46:465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi A, Jervis HR, Formal SB. 1975. Animal model of human disease. Bacillary dysentery, shigellosis, Shigella dysentery. Animal model: monkey shigellosis or dysentery. Am. J. Pathol. 81:251–254 [PMC free article] [PubMed] [Google Scholar]
- 13.Speelman P, Kabir I, Islam M. 1984. Distribution and spread of colonic lesions in shigellosis: a colonoscopic study. J. Infect. Dis. 150:899–903. 10.1093/infdis/150.6.899 [DOI] [PubMed] [Google Scholar]
- 14.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect. Immun. 74:790–793. 10.1128/IAI.74.1.790-793.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones FR, Hall ER, Tribble D, Savarino SJ, Cassels FJ, Porter C, Meza R, Nunez G, Espinoza N, Salazar M, Luckett R, Scott D. 2006. The New World primate, Aotus nancymae, as a model for examining the immunogenicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine 24:3786–3792. 10.1016/j.vaccine.2005.07.029 [DOI] [PubMed] [Google Scholar]
- 16.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 17.Verma NK, Lindberg AA. 1991. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine 9:6–9. 10.1016/0264-410X(91)90308-S [DOI] [PubMed] [Google Scholar]
- 18.Labrec EH, Schneider H, Magnani TJ, Formal SB. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 88:1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159:1126–1128. 10.1093/infdis/159.6.1126 [DOI] [PubMed] [Google Scholar]
- 20.Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, Hale TL. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 67:3437–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barzu S, Fontaine A, Sansonetti P, Phalipon A. 1996. Induction of a local anti-IpaC antibody response in mice by use of a Shigella flexneri 2a vaccine candidate: implications for use of IpaC as a protein carrier. Infect. Immun. 64:1190–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins TA, Barnoy S, Baqar S, Ranallo RT, Nemelka KW, Venkatesan MM. 2008. Safety and colonization of two novel VirG(IcsA)-based live Shigella sonnei vaccine strains in rhesus macaques (Macaca mulatta). Comp. Med. 58:88–94 [PMC free article] [PubMed] [Google Scholar]
- 23.Mills JA, Buysse JM, Oaks EV. 1988. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect. Immun. 56:2933–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaminski RW, Turbyfill KR, Oaks EV. 2006. Mucosal adjuvant properties of the Shigella invasin complex. Infect. Immun. 74:2856–2866. 10.1128/IAI.74.5.2856-2866.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turbyfill KR, Hartman AB, Oaks EV. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect. Immun. 68:6624–6632. 10.1128/IAI.68.12.6624-6632.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 27.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. 2009. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect. Immun. 77:1128–1136. 10.1128/IAI.01056-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karnell A, Cam PD, Verma N, Lindberg AA. 1993. AroD deletion attenuates Shigella flexneri strain 2457T and makes it a safe and efficacious oral vaccine in monkeys. Vaccine 11:830–836. 10.1016/0264-410X(93)90358-5 [DOI] [PubMed] [Google Scholar]
- 29.Kotloff KL, Herrington DA, Hale TL, Newland JW, Van De Verg L, Cogan JP, Snoy PJ, Sadoff JC, Formal SB, Levine MM. 1992. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect. Immun. 60:2218–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinari G, Hale TL, Austin SW, Formal SB. 1987. Local and systemic antibody responses to Shigella infection in rhesus monkeys. J. Infect. Dis. 155:1065–1069. 10.1093/infdis/155.5.1065 [DOI] [PubMed] [Google Scholar]
- 31.Rout WR, Formal SB, Giannella RA, Dammin GJ. 1975. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology 68:270–278 [PubMed] [Google Scholar]
- 32.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 164:533–537. 10.1093/infdis/164.3.533 [DOI] [PubMed] [Google Scholar]
- 33.Katakura S, Reinholt FP, Karnell A, Huan PT, Trach DD, Lindberg AA. 1990. The pathology of Shigella flexneri infection in rhesus monkeys: an endoscopic and histopathological study of colonic lesions. APMIS 98:313–319. 10.1111/j.1699-0463.1990.tb01038.x [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi A. 1982. Early colonic lesions in experimental Shigella infection in rhesus monkeys: revisited. Vet. Pathol. Suppl. 19(Suppl 7):1–8. 10.1177/030098588201907s01 [DOI] [PubMed] [Google Scholar]
- 35.Shipley ST, Panda A, Khan AQ, Kriel EH, Maciel M, Jr, Livio S, Nataro JP, Levine MM, Sztein MB, DeTolla LJ. 2010. A challenge model for Shigella dysenteriae 1 in cynomolgus monkeys (Macaca fascicularis). Comp. Med. 60:54–61 [PMC free article] [PubMed] [Google Scholar]
- 36.Katz DE, Coster TS, Wolf MK, Trespalacios FC, Cohen D, Robins G, Hartman AB, Venkatesan MM, Taylor DN, Hale TL. 2004. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect. Immun. 72:923–930. 10.1128/IAI.72.2.923-930.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]