FIG 5.
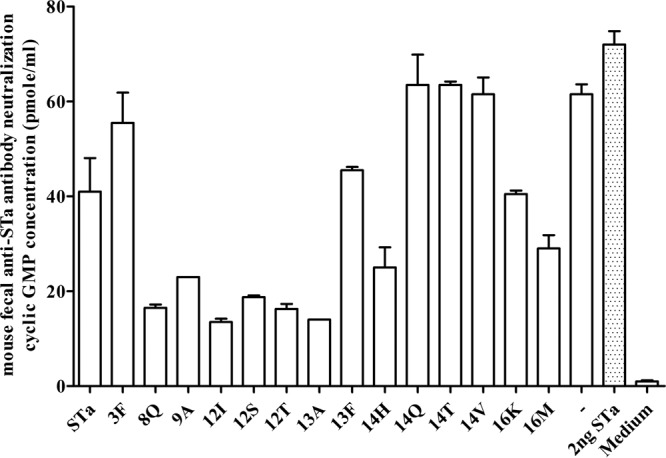
Mouse fecal suspension in vitro antibody neutralization activity against STa toxin, with intracellular cGMP levels in T-84 cells used to measure neutralizing activity of antibodies in fecal suspension samples against STa toxin. A fecal suspension sample (30 μl; in a final dilution of 1:200) pooled from each immunized group or the control group (5 mice per group) was incubated with STa toxin (2 ng; in 150 μl cell culture medium), and the fecal-toxin mixture was added to T-84 cells. The intracellular cGMP level in T-84 cells was measured by using an EIA cGMP ELISA kit (Assay Design), with columns and lines indicating the mean cGMP levels and standard deviations. A low cGMP level (lower than that of the control group) indicated antibody neutralizing activity against STa toxin. Group labels (on the x axis) indicate mice immunized with different fusion antigens and the control mice, for which fecal suspension samples were examined for STa neutralizing activity. STa toxin only (toxin without fecal sample) to show enterotoxicity in the stimulation of cGMP and cell culture medium (no STa toxin and anti-STa antibodies) to show the baseline cGMP level in T-84 cells were included as controls.
