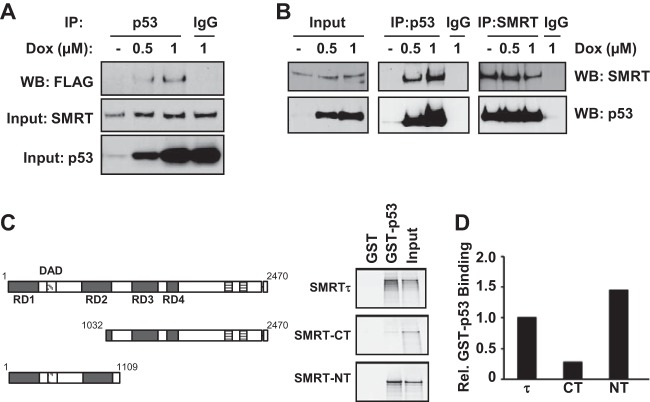
FIG 6.
SMRT binds to p53 both in vivo and in vitro. (A) MCF-7 cells were transfected with an expression vector for Flag epitope-tagged SMRT and treated with increasing concentrations of doxorubicin. Cell lysates were immunoprecipitated (IP) with p53 antibody or IgG, and immunoprecipitates were assessed by Western blotting with Flag antibody (top). Levels of SMRT (middle) and p53 (bottom) for input cell lysates were assessed by Western blotting with their respective antibodies. (B) HCT116 cells were treated with increasing doses of doxorubicin for 16 h, and total cell lysates were used for immunoprecipitation using antibodies for p53 or SMRT in parallel with the appropriate IgG negative control. Immunoprecipitated protein complexes were resolved by SDS-PAGE and analyzed by Western blotting using the indicated antibodies. (C) GST pulldown assays showing the interaction of p53 with full-length SMRTτ protein or its truncated mutants. Shown at the left are schematic representations of full-length SMRT and its truncated mutants. The bacterially expressed and purified GST-tagged p53 protein was incubated with in vitro-translated 35S-labeled SMRTτ or its truncated mutants, and the p53-bound SMRT or deletion mutants were detected by autoradiography. Input represents 20% of the corresponding amount of in vitro-translated 35S-labeled lysates. (D) Densitometric quantification of protein bands from panel C normalized to the amounts in the corresponding input lanes and represented relative to the amount of SMRTτ. Rel., relative.