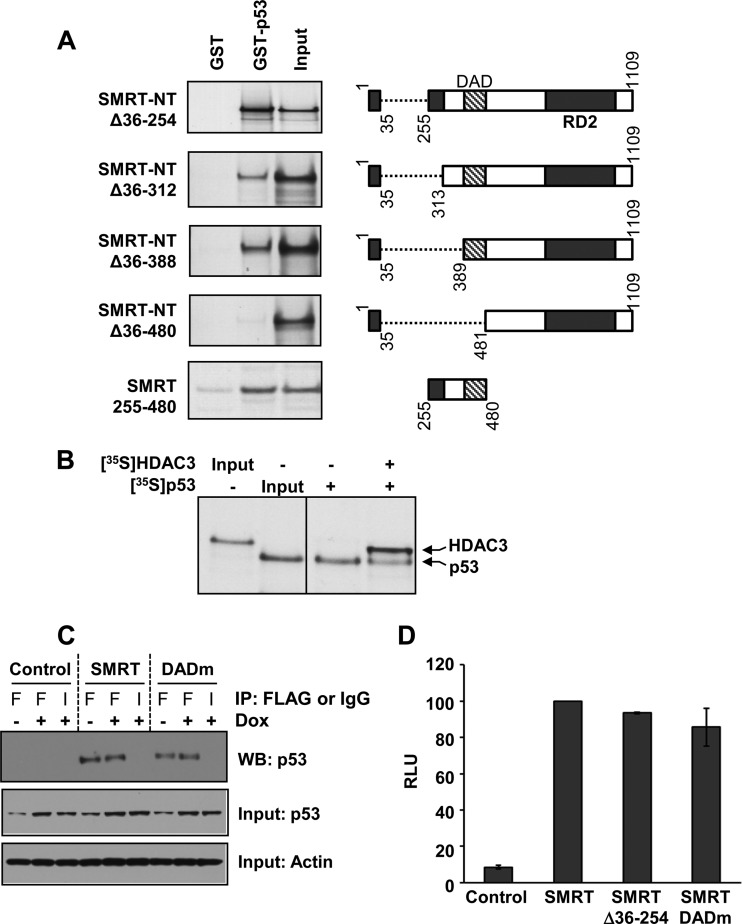
FIG 9.
The SMRT DAD binds to p53. (A) Bacterially expressed GST-tagged p53 protein was incubated with the in vitro-translated, 35S-labeled N terminus of SMRT (SMRT-NTΔ36-254), its deletion mutants, or the SMRT DAD fragment (residues 255 to 480) alone. Shown at the right are schematic representations of the SMRT deletion mutants and the DAD fragment. The protein complexes were immunoprecipitated by glutathione-agarose beads and resolved by SDS-PAGE, and the bound SMRT mutants or the DAD fragment was detected by autoradiography. Input represents 20% of the corresponding amount of the in vitro-translated, 35S-labeled lysates. (B) Competition between p53 and HDAC3 for binding to the SMRT DAD. Gal4-SMRT (DAD) was expressed in HCT116 cells and immobilized onto anti-Gal4 agarose beads to serve as bait. To this, in vitro-translated 35S-labeled p53 was added with or without in vitro-translated, 35S-labeled HDAC3. The protein complexes bound to agarose beads were resolved by SDS-PAGE, and the bound HDAC3 and p53 proteins were detected by autoradiography. Input represents 1 or 0.1% of the amount of in vitro-translated 35S-labeled lysates for HDAC3 and p53, respectively. (C) HCT116 cells were transfected with 1 μg expression vectors for control (pCR3.1) or Flag epitope-tagged SMRTτ or SMRT DADm and treated for 16 h with vehicle (−) or 0.5 μM doxorubicin (+). Cell lysates were subjected to immunoprecipitation using antibodies for Flag (lanes F) or an IgG negative control (lanes I). Immunoprecipitated protein complexes were resolved by SDS-PAGE and analyzed by Western blotting (WB) using p53 antibody (top). Relative input levels of p53 (middle) and actin (bottom) in the cell lysates were assessed by Western blot analyses. (D) HCT116 cells were transfected with 250 ng expression vectors for SMRTτ, SMRTΔ36-254, and SMRT DADm or an empty-vector control along with 1 μg of vector containing the p21-Luc reporter gene. Cells were harvested at 48 h after transfection, and luciferase activity was measured. The activity for the SMRT mutants is shown relative to that of SMRTτ. Values represent the averages ± SEMs (n = 2).