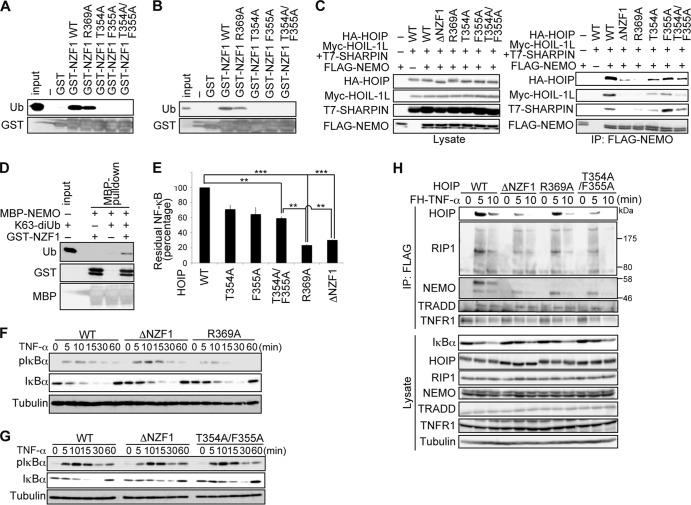
FIG 7.
Simultaneous recognition of NEMO and ubiquitin by HOIP NZF1 is required for NF-κB activation. (A and B) WT or mutant HOIP NZF1 fused to GST was incubated with K63 diubiquitin (A) or linear tetraubiquitin (B) as indicated, followed by pulldown with glutathione beads. Bound proteins were probed as indicated. (C) HA-HOIP or its mutants were transfected into HEK293T cells along with Myc-HOIL-1L, T7-SHARPIN, and FLAG-NEMO, and cell lysates (left) and anti-FLAG immunoprecipitates (right) were immunoblotted as indicated. (D) Full-length NEMO fused with MBP was incubated with K63-diubiquitin and GST-NZF1, followed by pulldown with maltose resins. Bound proteins were probed as indicated. (E) Luciferase activities in HEK293T cells expressing WT or mutant HA-HOIP, along with Myc-HOIL-1L, T7-SHARPIN, and 5× NF-κB luciferase reporter, are shown relative to the activity in cells expressing WT LUBAC, defined as 100% (means ± standard errors of the means; n = 3). **, P < 0.01; ***, P < 0.001 (Student's t test). (F and G) HOIP Δlinear MEFs retrovirally expressing WT, ΔNZF1, or R369A (F) or T354A/F355A (G) HOIP were treated with TNF-α (3 ng/ml) for the indicated periods and probed with the indicated antibodies. (H) HOIP Δlinear MEFs retrovirally expressing WT, ΔNZF1, R369A, or T354A/F355A HOIP were treated with FLAG-His6–TNF-α (FH-TNF-α) (3 μg/ml) for the indicated periods; cell lysates (bottom) and anti-FLAG immunoprecipitates (top) were immunoblotted as indicated.