FIG 6.
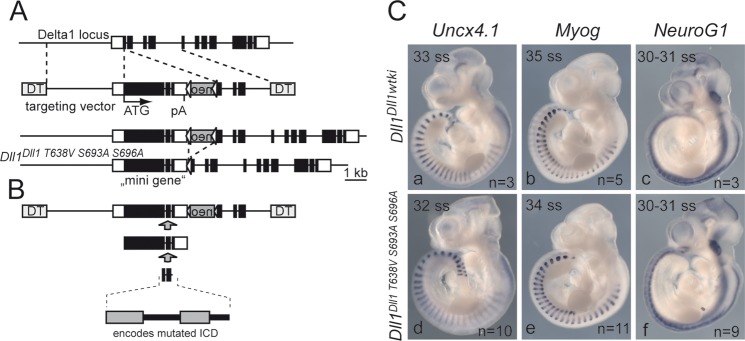
Targeting strategy and analysis of mutant embryos. (A) Schematic representation of targeting vector comprising a minigene, including 4 kb of the promoter region (providing the 5′ region of homology), exons 1 to 9 as cDNA followed by the remainder of the Delta1 gene (intron 9, exon 10, intron 10, and exon 11). After homologous recombination, the minigene replaces the coding portion of exon 1 and most of exon 2 analogously to our targeting strategy used to generate a lacZ knock-in allele of Delta1 (78), which has no apparent effect on the regulation of the Dll1 locus in vivo (61). The neo gene can be removed by Cre-mediated site-specific recombination. Coding exons are indicated by black boxes; the 5′ and 3′ untranslated regions are indicated by white boxes. Triangles flanking the neo gene indicate loxP sites. (B) A fragment encoding the intracellular domain with the three mutated phosphosites was synthesized and introduced into the targeting vector by conventional cloning. (C) Whole-mount in situ hybridization of wild-type and phosphosite mutant embryos. Markers for anterior-posterior somite patterning (Uncx4.1), myogenesis (MyoG), and neurogenesis (NeuroG1) were expressed indistinguishably in wild-type and mutant embryos. Shown are age-matched embryos at the indicated somite stage (ss). DT, diphtheria toxin A chain; pA, polyadenylation signal.
