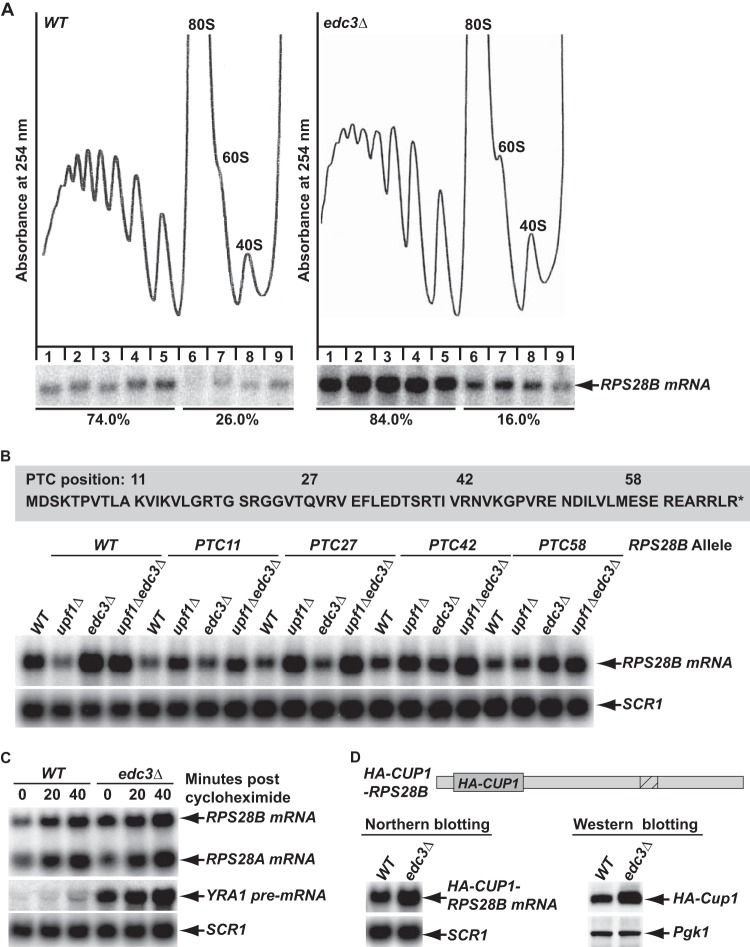
FIG 3.
Edc3-mediated RPS28B mRNA decay requires translation. (A) Analysis of the translation status of RPS28B mRNA in wild-type (HFY114) and edc3Δ (CFY25) cells. Wild-type and edc3Δ cells were grown in YEPD medium at 30°C, and whole-cell extracts were prepared. The polyribosomal association of RPS28B mRNA in these cells was analyzed by sucrose gradient fractionation and Northern blotting. (Top) Absorbance tracings at 254 nm. (Bottom) Northern blots of individual gradient fractions. The blots were hybridized with an oligonucleotide complementary to the RPS28B transcript. (B) RPS28B mRNA is sensitive to nonsense mutations introduced into its early coding region. Nonsense mutations were introduced into the indicated codon positions of the RPS28B gene. The resulting alleles were individually introduced into wild-type (CFY159), upf1Δ (CFY322), edc3Δ (CFY161), and upf1Δ edc3Δ (CFY324) strains with an rps28bΔ background. Total RNA was isolated from each of the resulting strains, and the steady-state levels of the RPS28B transcript encoded by each of the nonsense-containing alleles were analyzed by Northern blotting, using random-primed probes specific for the RPS28B or SCR1 transcripts. PTC, premature termination codon. (C) RPS28B mRNA is stabilized by treating cells with cycloheximide. Wild-type (HFY114) and edc3Δ (CFY25) cells were grown in YEPD medium at 30°C, and cycloheximide was added to the cell cultures. Cells were harvested at the indicated times. Total RNA was isolated from the collected cells, and the steady-state levels of RPS28B mRNA in the cells at each time point were analyzed by Northern blotting, using an oligonucleotide probe that hybridizes to both the RPS28A and RPS28B transcripts and a random-primed probe specific for the SCR1 transcript. (D) Analysis of mRNA and protein expression from the HA-CUP1-RPS28B reporter gene. The RPS28B coding region was replaced by HA-CUP1 coding sequences, and the resulting reporter gene was introduced into wild-type (HFY114) and edc3Δ (CFY25) cells. mRNA and protein expression from the reporter gene were analyzed by Northern or Western blotting, respectively. The Northern blots were hybridized to random-primed probes specific for the CUP1 and SCR1 transcripts, with the latter serving as a loading control. The Western blots were probed with monoclonal antibodies targeted to the HA epitope or Pgk1, with the latter serving as a loading control.