FIG 1.
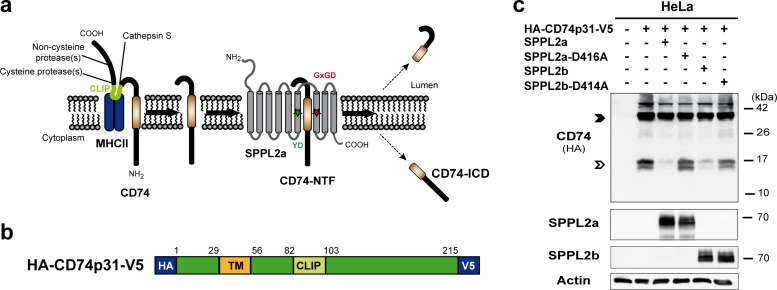
SPPL2b is capable of cleaving N-terminal fragments of CD74 in vitro. (a) Scheme of CD74-proteolysis in MHC-II compartments. The luminal domain of CD74 is sequentially degraded by several endosomal proteases. Finally, the proteolytic cleavage by cathepsin S releases the MHC-II dimer. Thereby, a small fragment (class II-associated li chain peptide [CLIP]) remains inside the MHC-II binding groove until it is replaced by an antigenic peptide. The N-terminal fragment of CD74 (NTF; 82 amino acids) is then further processed by the intramembrane protease SPPL2a. Colored asterisks indicate the catalytic motifs YD and GxGD within SPPL2a. (b) Layout of the utilized expression construct of the p31 isoform of murine CD74 with N- and C-terminally fused HA and V5 epitopes, respectively. TM, transmembrane segment. (c) HeLa cells were transiently transfected with CD74 alone or in combination with SPPL2a or SPPL2b or their catalytically inactive D416A or D414A mutant forms, respectively. Detection of CD74 was performed with an antibody against the N-terminally appended HA epitope tag after Western blotting and protein separation by SDS-PAGE using a Tris-Tricine buffer system with improved resolution in the low-molecular-weight range. Equal protein loading was confirmed by the detection of actin. Full-length CD74 and the CD74 NTF remaining after degradation of the luminal domain by endosomal proteases are marked with closed and open arrowheads, respectively.