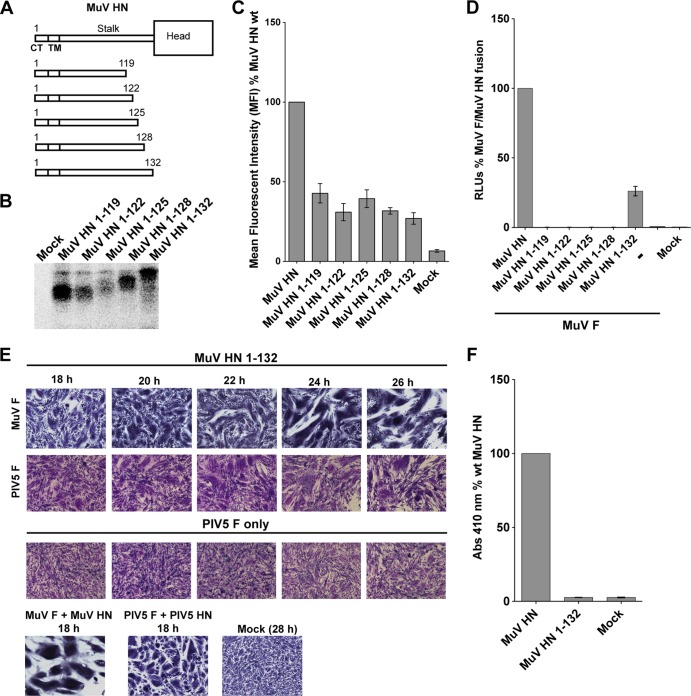
FIG 7.
A mumps virus HN “headless” stalk is able to trigger both cognate and noncognate F proteins. (A) Schematic representation of MuV wt HN protein and MuV HN “headless” stalks of various lengths. CT, cytoplasmic tail; TM, transmembrane domain. (B) Migration pattern of MuV HN “headless” stalk proteins on a SDS–15% PAGE gel. Polypeptides were immunoprecipitated from radiolabeled lysates of transfected 293T cells using the MuV polyclonal sera. (C) Expression of MuV HN “headless” stalk domains detected on the surface of transfected 293T cells using the MuV polyclonal sera. A goat α-rabbit FITC-conjugated secondary antibody was used for flow cytometry. The mean fluorescence intensities of the MuV HN “headless” stalks were expressed as a percentage of wt MuV HN surface expression (n = 3). (D) Luciferase reporter assay for fusion showing fusion promotion by the MuV HN “headless” stalk mutants cotransfected with MuV-F, expressed as a percentage of wt MuV-F and wt MuV HN fusion (n = 3). (E) In the first row, representative micrographs of syncytia show cell-cell fusion in BHK-21 cells transfected with MuV F and MuV HN 1-132 “headless” stalk at 18, 20, 22, 24, and 26 h posttransfection. For the second row, a syncytium assay was performed to show the ability of the MuV HN 1-132 “headless” stalk to cause cell-cell fusion when cotransfected with PIV5 F. Cells were fixed, stained, and photographed 18 to 26 h posttransfection. For the third row, a syncytium assay was performed to assess the expression of PIV5 F alone, so that spontaneous cell-cell fusion could be compared to fusion (26 h posttransfection) caused by cotransfection of MuV HN 1-132 and PIV5 F. (F) Receptor-binding ability of the MuV HN 1-132 mutant, as measured with a hemadsorption assay. The hemadsorption activity of the MuV HN 1-132 mutant was expressed as a percentage of the wt MuV HN activity (n = 3).