FIG 1.
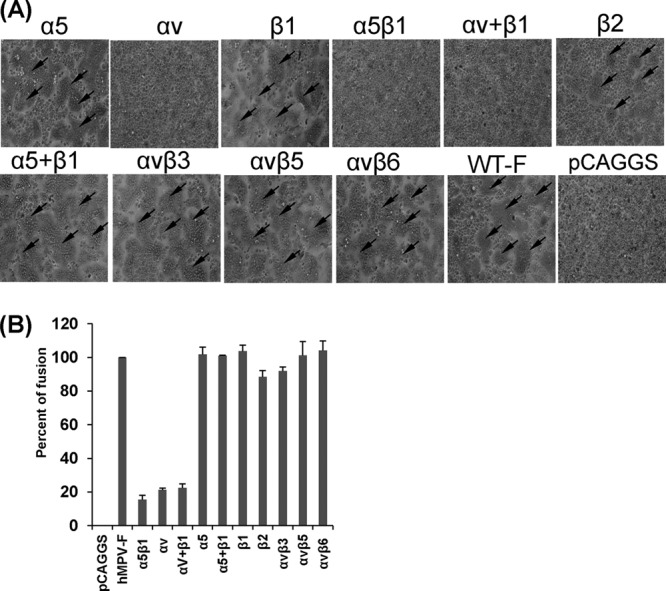
α5β1 and αv integrin-specific antibodies block cell-cell fusion triggered by hMPV F protein. (A) Syncytium formation induced by hMPV F protein expression in the presence of integrin antibodies. Vero E6 cells in 48-well plates were transfected with 0.8 μg of pCAGGS-F or pCAGGS. After incubation with a plasmid-Lipofectamine mixture for 8 h, cells continued to grow in Opti-MEM containing 10 μg/ml of integrin antibody and 0.2 μg/ml of TPCK-trypsin. At 48 h, monolayers were fixed with methanol and stained with Giemsa. Syncytia are indicated by arrows. (B) Content-mixing fusion assay in the presence of integrin antibodies. Vero E6 cells were cotransfected with 2 μg of pCAGGS-F and a reporter gene plasmid (pGINT7). At 24 h posttransfection, the cells were detached with trypsin and mixed with equal numbers of BHK-SR19-T7 cells, which express T7 RNA polymerase. Then, the cells were incubated with 2 ml of Opti-MEM containing 5 μg/ml of selected integrin antibody and 0.2 μg/ml of TPCK-trypsin for 12 h. The cells were lysed and mixed with an equal amount of the β-galactosidase substrate chlorophenol red-β-d-galactopyranoside (16 mM). The extent of fusion was quantitated by use of a microplate spectrophotometer at an absorbance of 570 nm. Percent fusion for each antibody treatment was normalized to the fusion of pCAGGS-F in the absence of integrin antibody. The data shown are averages for three independent experiments.
