FIG 1.
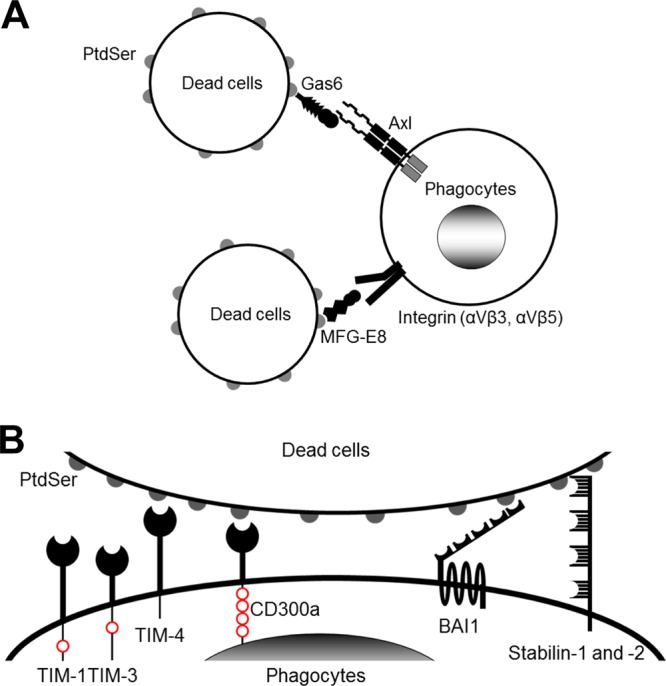
Molecular mechanisms of phagocytosis of dead cells. (A) Molecular mechanisms of phagocytosis of dead cells mediated by soluble molecules that bridge the PtdSer exposed on dead cells to receptors on phagocytes. (B) Molecular mechanisms of phagocytosis of dead cells mediated by receptors that directly recognize PtdSer. TIM-1, -3, and -4 and CD300a bind PtdSer via their N-terminal IgV domains. TIM-1 and -4 each have one cytoplasmic tyrosine residue and CD300a has four cytoplasmic tyrosine residues; these residues are phosphorylated and mediate signaling after binding to PtdSer (red circles). BAI1 is a seven-transmembrane receptor. Its extracellular N terminus has five thrombospondin type 1 repeat domains that bind PtdSer. The N termini of stabilin-1 and -2 each have four clusters of epidermal growth factor-like domain repeats that bind PtdSer. The cytoplasmic domains of BAI1 and stabilin-1 and -2 can activate Rac1 GTPase. In addition to these receptors, RAGE has recently been identified to bind PtdSer; however, its PtdSer-binding domain(s) has not yet been identified.
