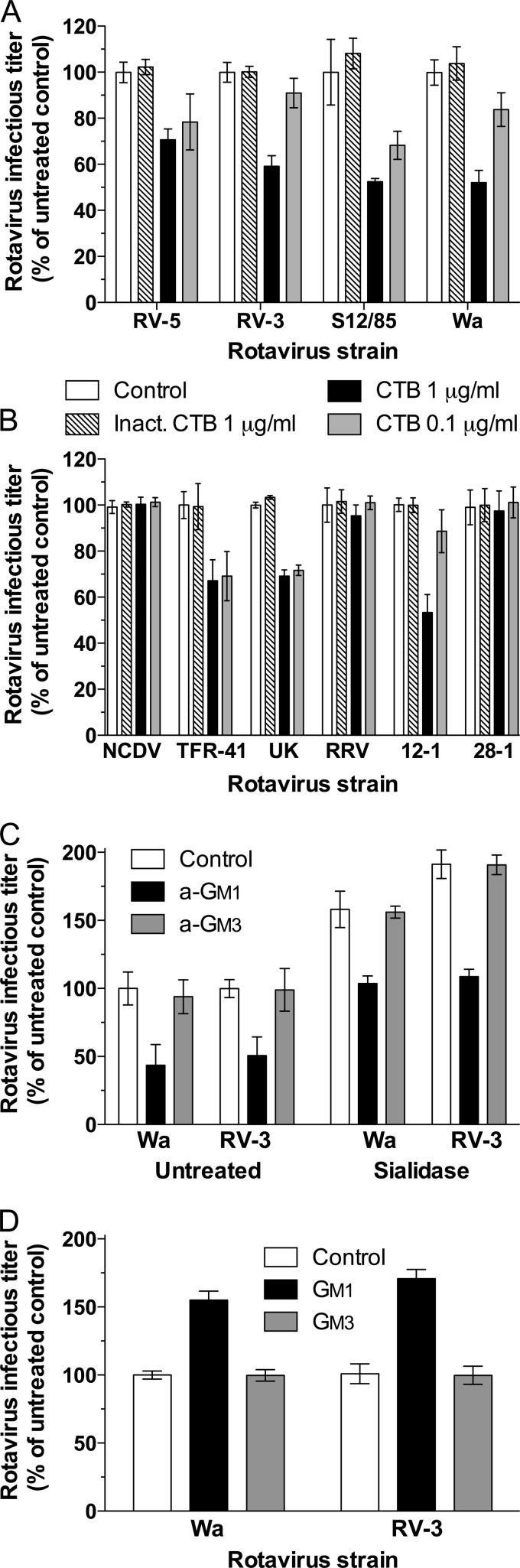
FIG 3.
Effects of CTB, a-GM1 treatment, and GM1 or GM3 supplementation on rotavirus infectivity in MA104 cells and mapping UK rotavirus dependence on GM1 to VP4. (A) CTB treatment inhibited infection by human rotaviruses RV-5, RV-3, S12/85, and Wa. (B) CTB inhibition of the infectivity of NCDV, TFR-41, UK, and RRV rotaviruses and reassortant rotaviruses 12-1 and 28-1. Heat-inactivated CTB (Inact.) was included as a negative control, as described before (31). (C) Effects of exposure to 10 mM a-GM1 or a-GM3 on the infectivity of Wa and RV-3 in untreated and sialidase-treated cells. (D) Effects of cell supplementation with 3 μM GM1 or GM3 on Wa and RV-3 infectivity. Rotavirus infectious titers are expressed as a percentage of the titers produced in control untreated cells. These control infectious titers (mean FCFU/ml ± SD) were 3.0 × 104 ± 0.1 × 104 (RV-5), 2.0 × 104 ± 0.1 × 104 (RV-3), 1.8 × 104 ± 0.3 × 104 (S12/85), 2.4 × 104 ± 0.1 × 104 (Wa), 2.6 × 104 ± 0.1 × 104 (NCDV), 2.6 × 104 ± 0.2 × 104 (TFR-41), 1.1 × 104 ± 0.1 × 104 (UK), 1.2 × 104 ± 0.2 × 104 (RRV), 2.7 × 104 ± 0.1 × 104 (12-1), and 7.9 × 103 ± 0.8 × 103 (28-1).