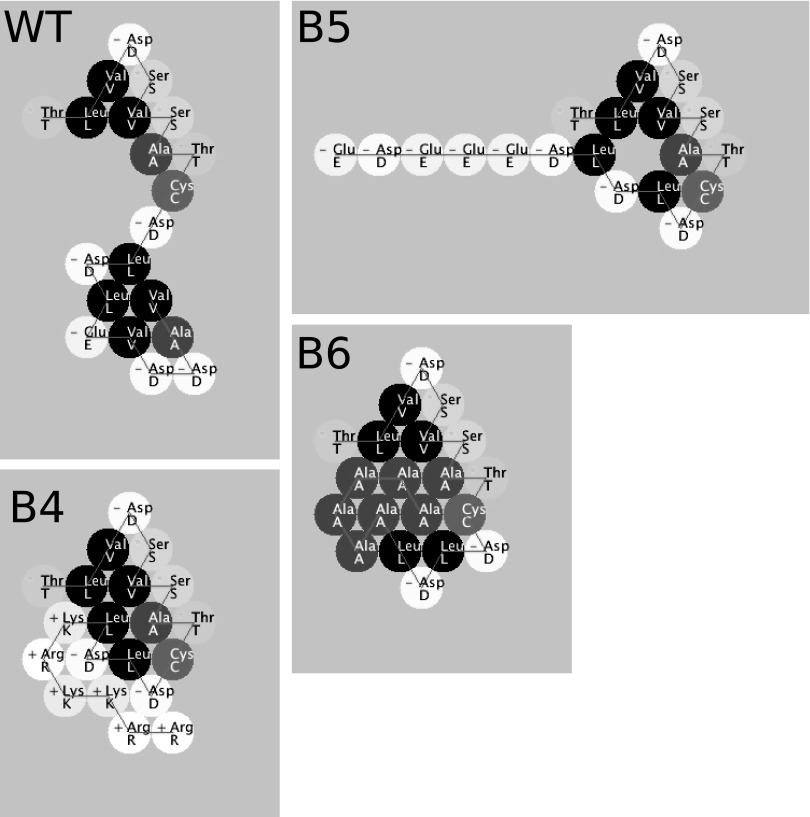
FIG 4.
Protein Investigator (ver. 3.0.2)-based prediction of folding of the C-terminal 20 amino acids of wild type (WT) pUL96 and predicted changes in protein folding as a result of the clustered mutations (B4, B5, and B6) that were introduced in this study. The above program folds the protein on the basis of the interactions between the side chains only and does not model secondary or quaternary structure. The circles are colored to indicate the relative hydrophobicities of the side chains. The most hydrophilic amino acids are white, the most hydrophobic amino acids are black, and gray amino acids are intermediate. Amino acids with positively charged side chains have a small plus sign on their symbol; those with negatively charged side chains have a small minus sign.