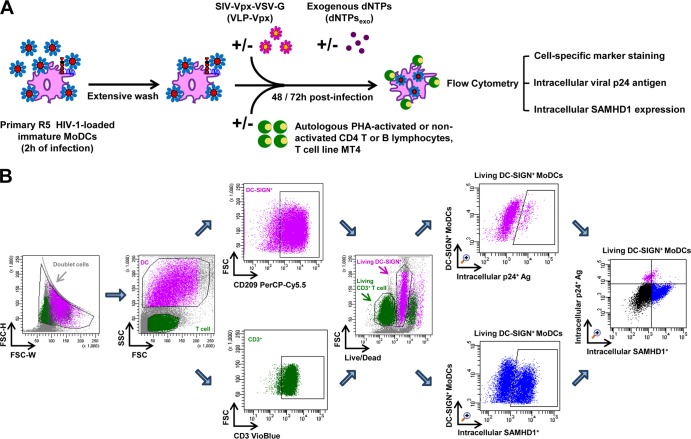
FIG 1.
Schematic representation of HIV-1 cell-to-cell transfer assay and the gating strategy for flow cytometry analysis. (A) HIV-1 cell-to-cell transfer assay. Immature MoDCs were pulsed with primary R5 HIV-1 isolates for 2 h and then thoroughly washed to remove unbound free viral particles. HIV-1-loaded MoDCs were incubated with or without autologous PHA-activated or nonactivated CD4 T or B lymphocytes or cells of the MT4 T cell line in 96-well plates. Where indicated, virus-like particles containing Vpx (VLP-Vpx) or exogenous dNTPs were added to infected MoDCs at the same time as lymphocytes as controls to decrease SAMHD1 levels and stimulate HIV-1 replication, respectively. After 48 and 72 h of culture, productive infection in MoDCs was quantified by detection of intracellular viral p24 antigen and intracellular SAMHD1 levels simultaneously by flow cytometry using cell-specific marker staining. (B) Quantification of productive infection and intracellular SAMHD1 levels by flow cytometry. Among all events, forward width and forward area were used to exclude doublet cells; forward angle and side scatter light gating were used to exclude cell debris. The DC population can easily be distinguished from lymphocytes according to size. Within the DC population, Ab directed against human CD209 (DC-SIGN, a DC-specific surface marker) was used to select DC-SIGN+ CD3− MoDCs; in the T cell population, Ab directed against human CD3 was used to select CD3+ CD4 T cells. Dead cells were then excluded with LIVE/DEAD fixable dead cell stain fluorescence kits. Percentages of living DC-SIGN+ MoDCs that are infected and contain intracellular SAMHD1 can be determined simultaneously. Double staining for HIV-1 infection and SAMHD1 expression in living DC-SIGN+ MoDCs is shown. The final analysis was performed with FACS Diva software, which generated a graphical output. FSC, forward scatter; SSC, side scatter.