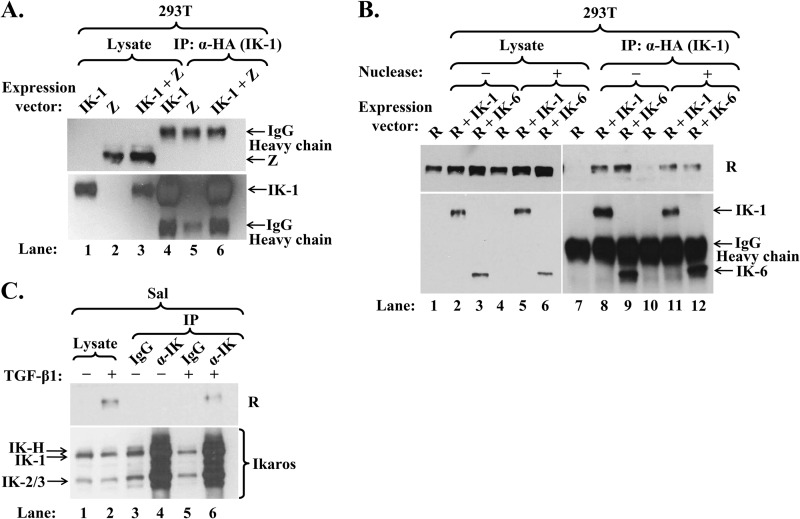
FIG 5.
Ikaros interacts with R but not Z. (A) Immunoblot showing failure of Z to coimmunoprecipitate with Ikaros. 293T cells in a 6-well plate were cotransfected with 0.06 μg p3xFLAG-Z and 0.2 μg pcDNA3-HA-IK-1 (IK-1 + Z) or either expression plasmid (Z or IK-1) plus empty vector pcDNA3.1. Whole-cell extracts were prepared 48 h later, and proteins were immunoprecipitated (IP) with an anti-HA-tag antibody. (B) Immunoblot showing coimmunoprecipitation of Ikaros isoforms and R. 293T cells in a 6-well plate were cotransfected with 0.1 μg pcDNA3-R and either 0.6 μg pCDH-EF1-HA-IK-6 (R + IK-6), 0.2 μg pCDH-EF1-HA-IK-1 plus 0.4 μg empty vector pCDH-EF1 (R + IK-1), or 0.6 μg empty vector pCDH-EF1 (R). Whole-cell extracts were prepared 48 h later and incubated for 20 min at room temperature with 800 U of Omnicleave endonuclease (Epicentre) per sample (+) or the same volume of dilution buffer (−) prior to processing as described in the legend for panel A. (C) Immunoblot showing coimmunoprecipitation of endogenous Ikaros and R. Sal cells were incubated for 72 h without (−) or with (+) TGF-β1 to induce EBV reactivation prior to preparation of whole-cell extracts and immunoprecipitation with anti-Ikaros or IgG antibody.