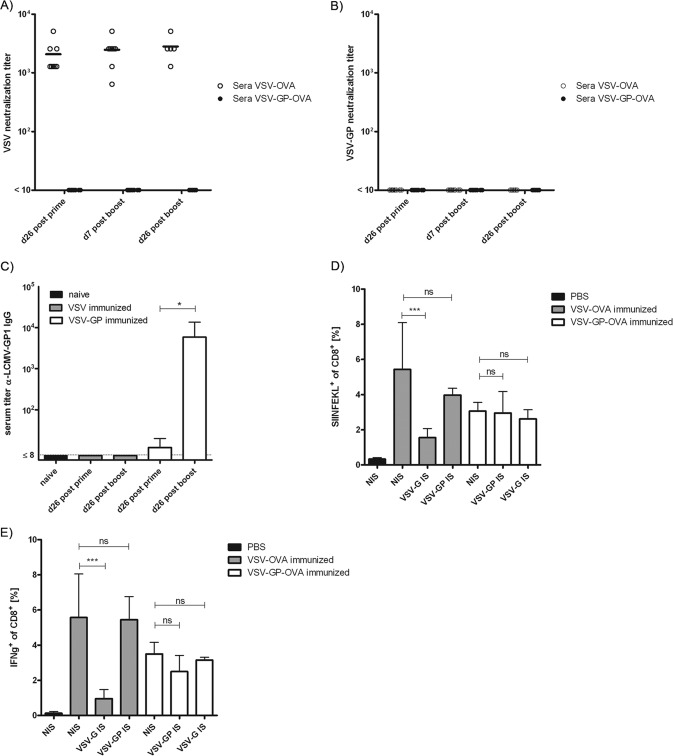
FIG 4.
Vaccination with VSV-GP does not induce neutralizing antibodies against the vector. Mice were immunized intramuscularly on days 0 and 26 with 1 × 106 PFU of VSV-OVA or VSV-GP-OVA in a prime/boost regimen, and plasma samples collected at the indicated time points were analyzed for the titer of neutralizing antibodies against VSV (A) or VSV-GP (B). Titers of neutralizing antibodies are given as the highest dilution which completely inhibited the cytopathic effect induced by 100 PFU of VSV-GFP or VSV-GP-GFP. At least 5 mice were analyzed per time point and virus. Plasma samples from VSV- or VSV-GP-immunized mice were collected at the indicated time points and were analyzed for the titer of LCMV-GP1 binding antibodies by ELISA using recombinant LCMV-GP1 (C). Plasma samples from 4 mice per group were pooled, and data show means ± standard deviations from 4 independent experiments. Statistical significances were determined using nonparametric statistics (two-tailed, Mann-Whitney test; *, ≤0.05). For serum transfer, naive mice received either immune serum from VSV-OVA- or VSV-GP-OVA-immunized mice or nonimmune serum from naive mice as a control. Subsequently, mice were immunized intramuscularly with 1 × 106 PFU of VSV-OVA or VSV-GP-OVA, and OVA-specific CTL responses were determined via tetramer (D) and intracellular cytokine (E) staining on day 7 postimmunization. Four mice per group were analyzed. Means ± standard deviations are shown. Statistical significances were determined using an unpaired, two-tailed t test (*, P ≤ 0.05; ***, P ≤ 0.01; ns, not significant).