ABSTRACT
Infection with wild-type measles virus (MeV) induces lifelong protection from reinfection, and parenteral delivery of the live attenuated measles vaccine (LAV) also provides protection from measles. The level of neutralizing antibody is a good indicator of protection, but the independent roles of MeV-specific antibody and T cells have not been identified. In this study, macaques immunized with LAV through a nebulizer and a mouthpiece developed MeV-specific T-cell responses but not neutralizing antibodies. Upon challenge with wild-type MeV, these animals developed rashes and viremias similar to those in naive animals but cleared viral RNA from blood 25 to 40 days faster. The nebulizer-immunized animals also had more robust MeV-specific CD4+ and CD8+ T-cell responses than the naive animals after challenge, characterized by a higher number and better durability of gamma interferon (IFN-γ)-producing cells. Induction of MeV-specific circulating CD4+ and CD8+ T cells capable of producing multiple cytokines correlated with clearance of viral RNA in the nebulizer-immunized macaques. These studies demonstrated that MeV-specific T-cell immunity alone did not prevent measles, but T-cell priming enhanced the magnitude, durability, and polyfunctionality of MeV-specific T cells after challenge infection and correlated with more rapid clearance of MeV RNA.
IMPORTANCE
The components of vaccine-induced immunity necessary for protection from infection and disease have not been clearly identified for most vaccines. Vaccine development usually focuses on induction of antibody, but T-cell-based vaccines are also under development. The live attenuated measles vaccine (LAV) given subcutaneously induces both T cells and neutralizing antibody and provides solid protection from infection. LAV delivered to the upper respiratory tract through a nebulizer and mouthpiece induced a T-cell response but no neutralizing antibody. These T-cell-primed macaques demonstrated no protection from rash or viremia when challenged with wild-type MeV, but viral RNA was cleared more rapidly than in unimmunized animals. Thus, T-cell immunity did not protect from infection or acute disease but facilitated virus clearance during recovery. These studies demonstrate the importance and independent roles of T cells and antibody in protection and recovery from measles.
INTRODUCTION
Vaccines play a vital role in preventing infectious diseases and have been developed to protect against many viral pathogens, but they are still needed to prevent infection with several emerging and persistent viruses (1). Most current successful vaccines were developed empirically with induction of antiviral antibody as a goal, but the actual determinants of vaccine-induced protection are complex and not fully characterized (2). Most viral vaccines are thought to provide protection from infection by inducing neutralizing antibody that prevents infection, but T-cell vaccines designed to eliminate virus-infected cells before dissemination are also in development (3–6). A more detailed understanding of the determinants of protective immunity and identification of the independent roles of virus-specific antibodies and T cells would inform the development of new vaccines and improvement of old vaccines. Identification of the underlying mechanisms of vaccine efficacy is most likely to be advanced by systematic evaluation of vaccine-induced immune responses combined with wild-type virus challenge in relevant animal models (7).
Measles is a systemic rash disease initiated in the respiratory tract by infection with measles virus (MeV). MeV infection of nonimmune hosts is characterized by viremia with rapid clearance of infectious virus but slow clearance of viral RNA (8), immune suppression (9–11), and a recovery process that results in lifelong immunity to reinfection (12). The live attenuated MeV vaccine (LAV) was developed by adaptation of a wild-type isolate of MeV to growth in tissue culture and has been highly successful in measles control (13). The virus particle contains 6 proteins: the surface glycoproteins hemagglutinin (H) and fusion protein (F), which mediate attachment and entry; and the internal proteins nucleocapsid (N), matrix (M), phosphoprotein (P), and polymerase (L). Two nonstructural proteins, C and V, regulate host responses to infection (14). Immune responses are induced to most of these viral proteins (15–18).
Antibody to H protein is most important for virus neutralization (19), and CD4+ and CD8+ T-cell epitopes are present in most proteins (16–18). Epidemiological studies have shown that the level of neutralizing antibody at the time of exposure is a good indicator of protection (20), but T cells have also been implicated as protective in individuals with low levels of antibody (21). Therefore, the specific components or combination of components of the immune response induced by prior infection or vaccination actually responsible for protection are not known. In particular, the role of T cells is poorly defined.
The antiviral effects of T cells can be mediated both by secretion of cytokines that suppress virus replication and by cytotoxic elimination of infected cells (22–24). Because T cells do not directly block infection but rather react to control or eliminate virus-infected cells once infection has occurred (25), the contribution of T cells to vaccine-mediated protection is generally considered minor in comparison to that of neutralizing antibodies. However, induction of broadly effective neutralizing antibodies has been difficult to achieve for viruses with large numbers of serotypes and rapid mutation of surface proteins (26–28), and in young infants, residual maternal antibodies interfere with induction of MeV-specific antibody by LAV (29–31). In these situations, it would be useful to develop vaccines that induce protective T-cell responses to more conserved viral proteins.
In this study, we have taken advantage of experiments designed to explore delivery of LAV by the respiratory route using a variety of delivery devices (32). While aerosol delivery of dry powder LAV as small particles induced both T-cell and neutralizing antibody responses (32), macaques given liquid LAV through a nebulizer and a mouthpiece developed a T-cell response but not neutralizing antibodies. By comparing the virologic and immunologic responses to wild-type MeV challenge of nebulizer-immunized macaques with the responses of unimmunized naive macaques, we were able to identify the contributions of preexisting T-cell immunity to protection from measles.
RESULTS
Unique immune responses were induced after respiratory measles vaccination.
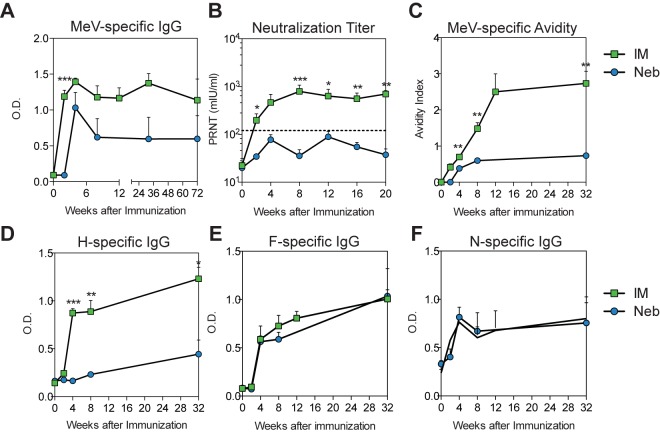
During experiments aimed at developing a needleless platform for the delivery of measles vaccines (32), three macaques were immunized with a single dose of the live attenuated Edmonston-Zagreb (EZ) measles vaccine through a nebulizer and a standard mouthpiece (see Fig. S1 in the supplemental material). After immunization, these macaques developed MeV-specific T-cell responses to both the H and F glycoproteins comparable to those observed in macaques that received the same vaccine by intramuscular (i.m.) injection (Fig. 1A and B). However, the antibody responses of macaques in the nebulizer-immunized group were poor (Fig. 2). Titers of MeV binding antibody (Fig. 2A) and neutralizing antibody (Fig. 2B) and avidity of MeV binding antibody (Fig. 2C) were significantly lower for nebulizer-immunized animals. Examination of the specificity of the antibody demonstrated that the defect in the humoral response in nebulizer-immunized macaques was specific for the induction of antibody to H (Fig. 2D), while comparable levels of F- and N-specific antibodies were induced (Fig. 2E and F). Because antibody to H accounts for the majority of the neutralizing antibody (19, 33), low H-specific antibody explains the low titers of neutralizing antibody in these animals.
FIG 1 .

MeV-specific T-cell responses after nebulized (Neb) and intramuscular (IM) immunization with LAV. T-cell responses were assessed by IFN-γ ELISpot assays. PBMCs were stimulated with overlapping peptides from the hemagglutinin (H) (A) or fusion (F) (B) proteins. Numbers of MeV-specific spot-forming cells (SFCs) were determined by averaging spots in duplicate peptide-stimulated wells and subtracting spots in unstimulated wells. Data are presented as average numbers of SFCs/106 PBMCs + the standard error of the mean (SEM) for each group (n = 3). No significant differences were detected (H-specific responses, 2 weeks, P = 0.13; 4 weeks, P = 0.35; Student’s t test).
FIG 2 .
MeV-specific antibody responses after nebulized (Neb) and intramuscular (IM) immunization with LAV. Total MeV-specific IgG (A) and MeV H-, F-, and N-specific IgG (D to F) were determined by enzyme immunoassays using plates coated with lysates from MeV-infected Vero cells (A), MeV H-expressing L cells (D), MeV F-expressing L cells (E), or baculovirus-expressed MeV N (F). Values are plotted as means + SEM of optical density (OD). (B) Neutralizing antibody determined by plaque reduction neutralization test. The predicted protective level of antibody (120 mIU/ml) is indicated with a dashed line. Values are plotted as geometric means + SEM. (C) Avidity of MeV-specific IgG assessed by disruption of antibody binding with 0.5 to 3.0 M ammonium thiocyanate (NH4SCN). Values are plotted as means ± SEM of the avidity index, calculated as the concentration of NH4SCN at which 50% of the bound antibody was eluted. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (Student’s t test).
Priming a T-cell response without neutralizing antibody to MeV did not protect animals from disease.
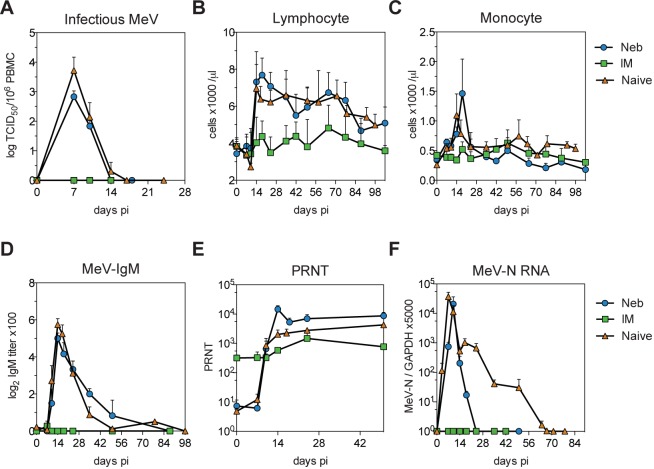
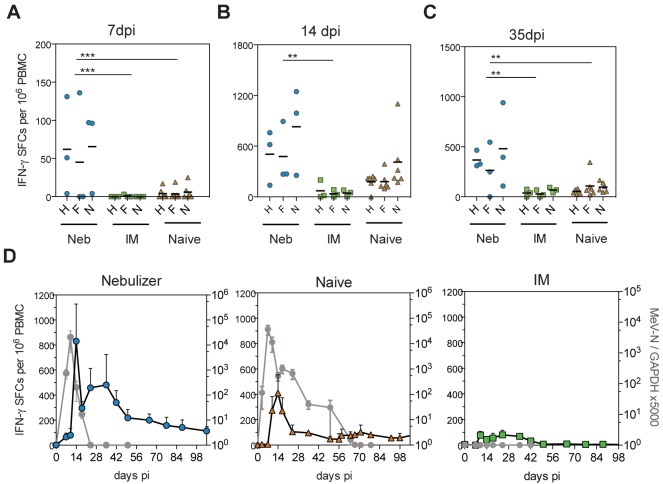
To determine whether priming a T-cell response to MeV without inducing neutralizing antibody was sufficient to provide protection from subsequent wild-type MeV infection, vaccinated macaques, along with control naive macaques, were infected intratracheally with 104 50% tissue culture infective doses (TCID50) of the Bilthoven strain of wild-type MeV 17 months after vaccination (Fig. 3). Macaques in the i.m.-immunized group were completely protected from rash, viral shedding, and viremia (Fig. 3A and Table 1). Two out of three macaques in the nebulizer-immunized group developed a rash (Table 1), and all had viremias with titers comparable to those of the naive macaques (Fig. 3A) and changes in the numbers of circulating lymphocytes and monocytes typical of measles (34) (Fig. 3B and C). However, shedding from the respiratory tract was more transient, with viral RNA detected in two of three nebulizer-immunized animals only on day 14, while RNA was detected in all naive animals on multiple days, beginning on day 7 or 10 (Table 1).
FIG 3 .
Viremia, blood leukocyte counts, antibody responses, and levels of viral RNA after wild-type MeV challenge. (A) Infectious virus in the blood quantified by cocultivation of PBMCs with B95-8 or Vero/hSLAM cells. Infectious MeV was not detected in i.m.-immunized (IM) animals but was cleared by 14 days in nebulizer-immunized (Neb) animals and by 17 days in naive macaques. TCID50, 50% tissue culture infectious dose. Differences between the naive and nebulizer-immunized groups were not significant. (B and C) Absolute numbers of circulating lymphocytes and monocytes. (D) MeV-specific IgM determined by enzyme immunoassay using plates coated with MeV-infected Vero cell lysate. IgM was detected in the nebulizer-immunized and naive animals but not in i.m.-immunized animals. (E) Neutralizing antibody determined by plaque reduction neutralization test (PRNT). Values are plotted as geometric means + SEM. Titers were higher in nebulizer-immunized than naive and i.m.-immunized animals on day 14 (nebulizer immunized versus naive, P < 0.05; nebulizer immunized versus i.m. immunized, P < 0.01). Titers were higher in i.m.-immunized than in nebulizer-immunized and naive animals on days 0 and 7 (P < 0.001, two-way ANOVA with Bonferroni posttest). (F) MeV RNA quantified by qRT-PCR on total RNA extracted from 2 × 106 PBMCs. RNA was amplified with MeV N-specific primers and measured with an N-specific probe. Results were normalized to GAPDH. No MeV RNA was detectable in i.m.-immunized macaques. MeV RNA was cleared by 24 days in nebulizer-immunized macaques and by 70 days in naive macaques. Values are shown as means + SEM.
TABLE 1 .
Detection of rash and MeV RNA in nasal swab samples
| Immunization group | Macaque | Rash | Viral shedding on day: |
|||||
|---|---|---|---|---|---|---|---|---|
| 7 | 10 | 14 | 17/18 | 23/24 | 35 | |||
| Nebulizer | 1T | No | − | − | + | − | − | − |
| 18T | Yes | − | − | + | − | − | − | |
| 22T | Yes | − | + | + | + | + | − | |
| i.m. | 38T | No | − | − | − | − | − | − |
| 47T | No | − | − | − | − | − | − | |
| 67T | No | − | − | − | − | − | − | |
| Naive | 15U | Yes | + | + | + | − | − | − |
| 46U | Yes | − | + | + | − | − | − | |
| 55U | Yes | + | + | + | + | − | − | |
| 67U | Yes | + | + | + | + | + | + | |
| 40V | Yes | + | + | − | − | − | NAa | |
| 43V | Yes | + | + | + | + | − | NA | |
NA, not available.
Both naive and T-cell-primed nebulizer-immunized macaques developed a similar robust IgM response, indicating a primary antibody response to MeV challenge infection (Fig. 3D). The time courses for the development of neutralizing antibody after challenge were also similar between naive and nebulizer-immunized macaques (Fig. 3E), although nebulizer-immunized animals had higher titers than the naive and i.m.-immunized animals on day 14 (nebulizer immunized versus naive, P < 0.05; nebulizer immunized versus i.m. immunized, P < 0.01). Taken together, these data show that in the absence of neutralizing antibody and H-specific B-cell memory, T cells induced by measles vaccination alone did not protect from MeV infection or systemic virus spread. However, induction of local immunity may have decreased virus shedding from the upper respiratory tract.
Priming a T-cell response to MeV accelerated the clearance of MeV RNA.
Although MeV is a classic example of an acute infection with rapid recovery and clearance of infectious virus, we have previously demonstrated that the prolonged presence of viral RNA in blood and lymphoid tissues after resolution of the rash is characteristic of primary MeV infection in humans and macaques (8, 35, 36). We thus examined the impact of T-cell priming on MeV RNA clearance (Fig. 3F). Initial loads of infectious MeV (Fig. 3A) and viral RNA (Fig. 3F) were similar between macaques in the nebulizer-immunized and naive animals, but MeV RNA clearance was faster in the nebulizer-immunized group. MeV RNA was detected in the blood of nebulizer-immunized macaques for a median of 18 days and was detected in naive macaques for a median of 50 days (range, 24 to 67 days) (Fig. 3F). Therefore, priming a T-cell response to MeV did not protect macaques from measles but did accelerate clearance of viral RNA.
Magnitude and durability of the MeV-specific T-cell response correlated with the control of MeV RNA after challenge.
To investigate the relationship between rapid clearance of MeV RNA and the cellular immune response, MeV-specific gamma interferon (IFN-γ)-producing T cells were quantified after wild-type MeV challenge using an enzyme-linked immunospot (ELISpot) assay (Fig. 4). Macaques in the nebulizer-immunized group had more rapid production of MeV-specific T cells than naive macaques, with detection at 7 days after infection in the nebulizer-immunized but not the naive group (mean numbers of spot-forming cells [SFCs], nebulizer immunized, 57.6; naive, 4.6; P < 0.001) (Fig. 4A). In addition, the number of IFN-γ-producing T cells at the peak of the response on day 14 was higher in the nebulizer-immunized macaques than in naive or i.m.-immunized macaques (mean numbers of SFCs, nebulizer immunized, 604; i.m. immunized, 50; naive, 256) (Fig. 4B). Therefore, vaccine-induced T-cell priming generated T-cell memory that produced an efficient recall response to wild-type MeV challenge. T-cell priming also affected the durability of MeV-specific T-cell immunity after challenge with a higher number of IFN-γ-producing cells in the nebulizer group than the naive group 35 days after infection (mean numbers of SFCs, nebulizer immunized, 371; naive, 86; P < 0.01) (Fig. 4C). Examination of the time course of the N-specific T-cell response in relation to MeV RNA load showed an inverse relationship between T-cell numbers and clearance of viral RNA (Fig. 4D). Macaques in the naive group had a waning T-cell response and persistence of MeV RNA, while those in the nebulizer-immunized group had prolonged circulation of MeV-specific T cells with a rebound 14 to 50 days after infection and clearance of MeV RNA. Therefore, the MeV-specific T-cell response in naive animals was later and more transient than in nebulizer-primed animals. In naive animals, the MeV-specific T-cell response correlated with clearance of infectious virus but did not lead to clearance of MeV RNA, while in primed animals, the T-cell response was prolonged and both infectious virus and viral RNA were rapidly cleared from peripheral blood mononuclear cells (PBMCs).
FIG 4 .
MeV-specific T-cell responses after challenge with wild-type MeV. Shown are T-cell responses 7 (A), 14 (B), and 35 (C) days after challenge as assessed by IFN-γ ELISpot. PBMCs were stimulated with overlapping peptides from the hemagglutinin (H), fusion (F), or nucleocapsid (N) proteins. Numbers of MeV protein-specific spot-forming cells (SFCs) were calculated by averaging duplicate wells and subtracting nonspecific responses. Nebulizer-immunized macaques had a more robust T-cell response than i.m.-immunized (IM) or naive macaques 14 and 35 days after infection (one-way ANOVA with Bonferroni’s multiple comparison tests). ***, P < 0.001; **, P < 0.01; *, P < 0.05. (D) Correlation of the dynamics of the N-specific T-cell response and MeV RNA load. Shown is the group average of the number of N-specific SFCs (blue, orange, and green symbols) plotted against MeV N RNA load in PBMCs (gray symbols). Nebulizer-immunized animals showed a biphasic pattern of IFN-γ-producing T cells with a first peak at 14 days and a second peak at 35 days after challenge. The MeV RNA load and magnitude of T-cell responses showed an inverse relationship.
Quality of MeV-specific T-cell responses correlated with clearance of MeV RNA.
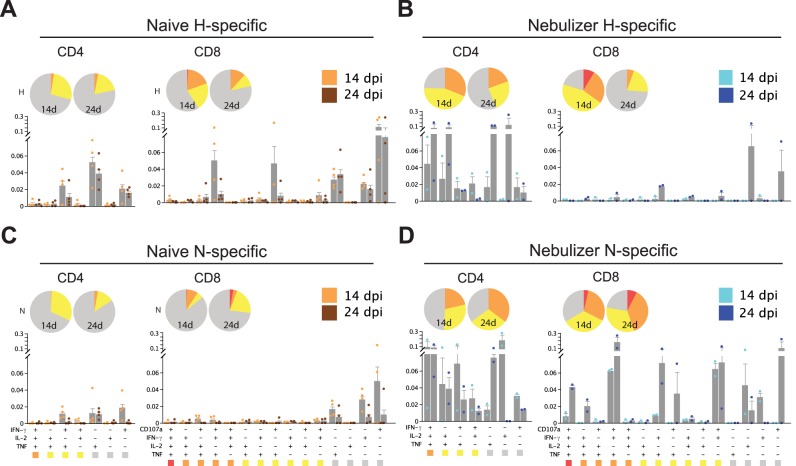
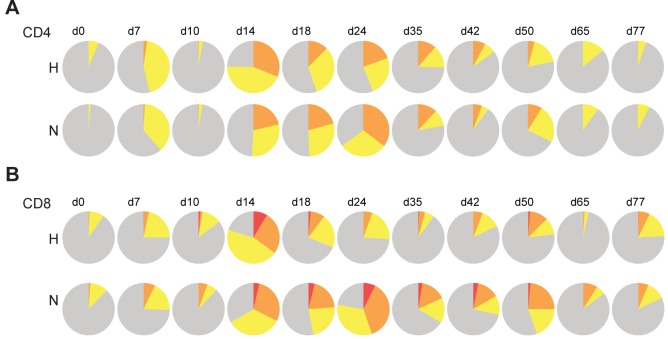
Because the quality as well as the quantity of the T-cell response is likely to be important for controlling virus infection (37–39), we assessed the ability of MeV-specific T cells to produce effector cytokines at different times after infection using intracellular cytokine staining and multiparameter flow cytometry (Fig. 5). In naive animals, even at the peak of the T-cell response (day 14), only a small fraction of H-specific (Fig. 5A) or N-specific (Fig. 5C) T cells were polyfunctional (CD4+ T cells capable of producing IFN-γ, tumor necrosis factor [TNF], and interleukin-2 [IL-2] or CD8+ T cells capable of expressing IFN-γ, TNF, IL-2, and CD107a simultaneously). However, nebulizer vaccine-induced T-cell priming substantially improved polyfunctionality of MeV-specific CD4+ and CD8+ T cells produced in response to challenge. More polyfunctional H- and N-specific CD4+ and N-specific CD8+ T cells were present in the nebulizer-immunized animals than naive animals when assessed by either absolute percentage (bar graph) or relative fraction of all functional categories (pie charts in Fig. 5B and D; see Fig. S2 in the supplemental material). To determine whether T-cell priming improved the durability of the polyfunctional T-cell response, we analyzed CD4+ and CD8+ T cells 24 days after infection, when the levels of viral RNA differed between the groups (Fig. 3F). Similar to data from day 14, more polyfunctional CD4+ and CD8+ T cells were present in the nebulizer-immunized group at day 24.
FIG 5 .
Functionality of MeV-specific T cells after challenge of nebulizer-immunized and naive macaques with wild-type MeV analyzed by intracellular cytokine staining and multicolor flow cytometry. A total of 106 fresh PBMCs were stimulated with medium or pooled H (A and B) or N (C and D) peptides (1 µg/ml) in the presence of anti-CD107a antibody, brefeldin A, and Golgistop for 12 h. Cells expressing IFN-γ, TNF, IL-2, or CD107 were gated from CD3+ CD4+ cells and CD3+ CD8+ cells. Subsets of cells expressing each functional marker were analyzed by Boolean gating. Seven CD4+ subsets and 15 CD8+ subsets were identified. Subsets that express one (gray), two (yellow), three (orange), or four (red) different functional markers were grouped. The frequency of each subset within CD4+ or CD8+ T cells was calculated by subtracting nonspecific responses and averaging results from animals in the naive (A and C; orange) or nebulizer-immunized (B and D; blue) groups and shown in the bar chart. The functional composition of CD4+ and CD8+ T-cell responses is shown in pie charts. More polyfunctional H- and N-specific CD4+ and N-specific CD8+ T cells were present in nebulizer-immunized than naive macaques.
Because the dynamics of polyfunctional T-cell production have not been studied over an extended period of time after acute virus infection, we analyzed MeV-specific polyfunctional T cells in nebulizer-immunized animals for 11 weeks (Fig. 6). N-specific polyfunctional CD4+ and CD8+ T cells present 14 days after challenge were stably maintained through 24 days and then waned gradually. H-specific polyfunctional CD4+ T cells followed a similar course, but H-specific polyfunctional CD8+ T cells were more transient.
FIG 6 .
Longitudinal analysis of the functionality of MeV-specific T cells in nebulizer-immunized macaques after infection. PBMCs from nebulizer-immunized animals collected at various times after wild-type MeV challenge were stimulated with medium or with pooled H or N peptides. Subsets that simultaneously express one (gray), two (yellow), three (orange), or four (red) different functional markers were grouped. Each pie chart shows the functional composition of the CD4+ and CD8+ T-cell responses at a given time point. Polyfunctional N-specific CD4+ and CD8+ T cells and H-specific CD4+ T cells were stably maintained between 14 and 24 days postinfection (dpi) and then waned gradually, while polyfunctional H-specific CD8+ T cells waned more rapidly.
DISCUSSION
This study has shown that a vaccine-induced T-cell response to MeV did not prevent MeV infection or disease but contributed to more rapid control of MeV RNA after infection. T-cell priming-associated accelerated clearance of viral RNA was accompanied by improved magnitude, durability, and polyfunctionality of MeV-specific T cells after challenge. The association of an enhanced T-cell response with rapid control of MeV RNA suggests that failure of rapid MeV RNA clearance after primary infection in naive animals may be due to inadequate function or insufficient proliferation of MeV-specific effector T cells during the acute phase of infection.
Respiratory delivery of aerosolized LAV has been an attractive approach to improving measles vaccine coverage for several decades, but it has never been licensed or widely used in part because of variable or suboptimal antibody responses (40, 41). In the present study, macaques immunized with LAV through a nebulizer developed an MeV-specific T-cell response and antibodies to F and N but did not develop antibodies to H. Although antibody to F can be neutralizing (19), the anti-F antibodies induced in these animals did not have detectable neutralizing activity and were not protective. A divergence of T-cell responses and antibody responses to H has not been observed after conventional i.m. or subcutaneous injection of LAV and was not observed after deep lung delivery of dry powder LAV (32). However, low or transient antibody responses to H in the presence of responses to F have been observed previously in monkeys immunized with a formalin-inactivated measles vaccine (42) and with LAV engineered to express IL-12 (43). Effects of differential priming of responses to individual MeV proteins have also been observed with experimental vaccines. For instance, DNA vaccines expressing only H prime for a Th2 response, while vaccines expressing only F prime for a Th1 response after challenge (44). Our results suggest that the route of immunization, including regional localization in the respiratory tract, matters for LAV-induced immunity. Furthermore, the requirements for generating high-quality H-specific antibody are different from the requirements for generation of H-specific T cells or for generation of antibody responses to F and N. This is further suggested by data on the specificity of polyfunctional T cells where the N-specific CD8+ T cells were more durable than H-specific CD8+ T cells.
In unimmunized individuals, the cellular immune response appears to play a more important role in controlling MeV infection than the humoral response. Patients with B-cell deficiency generally recover from measles, while those with T-cell defects may fail to clear the virus and develop progressive disease (45, 46). A role for T cells in containing primary MeV infection was further demonstrated by studies of macaques selectively depleted of either B cells or CD8+ T cells (47, 48). Replication of MeV was greater and the rash was prolonged in the absence of CD8+ T cells but was not affected by depletion of B cells. However, immune responses required for protection from infection are not necessarily the same as those required for recovery from infection. The present studies are the first to experimentally demonstrate that MeV-specific T cells do not provide protection from infection but do facilitate recovery from infection.
MeV is the classic example of an acute infection that induces lifelong immunity, and we have previously demonstrated that prolonged RNA presence in blood and lymphoid tissues is characteristic of primary MeV infection in humans and macaques (8, 35, 36). A mathematical model that expressed viral replication and elimination in terms of the strength of MeV-specific T-cell responses, antibody responses, target cell limitations, and regulatory T-cell immunosuppression indicated that T cells alone were insufficient to eliminate viral RNA and that antibody was required. Overall, these studies of MeV infection of naive macaques suggest that rapid waning of MeV-specific T-cell activity, potentially as a result of MeV-induced immune suppression, may account for the prolonged presence of MeV RNA. In the present study, improved T-cell responses through nebulizer priming, with higher magnitude, greater frequency, and prolonged maintenance of polyfunctional T cells, were associated with rapid clearance of MeV RNA. Collectively, our data suggest the possibility that MeV-induced immune suppression affects both global and MeV-specific T-cell responses and results in failure of the host to clear MeV RNA during the acute phase of infection. However, prolonged stimulation of the immune response by persistent MeV may also facilitate maturation of the antibody response and lifelong immunity, an effect that can perhaps be substituted for by priming the CD4+ T-cell response.
Identification of the role of specific functional capacities of T cells in controlling and clearing infection and for providing protection from infection is an area of importance for understanding pathogenesis of viral diseases, as well as the effectiveness of vaccines (1, 8). Despite the limitations of a small sample size and potential variability between outbred animals, our work has provided new insights into the role of vaccine-induced T cells in facilitating recovery from acute viral infection in a highly relevant animal model to human measles. These findings shed light on the likely limitations of T-cell-based vaccines for measles and potentially for other viral diseases in need of vaccine development.
MATERIALS AND METHODS
Animals.
Twelve 1- to 2-year-old MeV-naive rhesus macaques (Macaca mulatta) from the Johns Hopkins Primate Breeding Facility were studied. All monkeys were anesthetized with ketamine (10-15 mg/kg) during procedures. Animals were maintained and studies were performed in accordance with experimental protocols approved by the Johns Hopkins University Animal Care and Use Committee.
Immunization and MeV challenge.
Rhesus macaques were vaccinated with a single dose (1,000 PFU) of liquid Edmonston-Zagreb (EZ) live attenuated MeV vaccine generated by a no. 646 nebulizer and Pulmo Aid compressor (DeVilbiss Health Care) through a standard mouthpiece (n = 3; macaques 1T, 18T, and 22T) (see Fig. S1 in the supplemental material) or by i.m. injection (n = 3; macaques 38T, 47T, and 67T). Heparinized blood was collected for evaluation of the immune responses.
For MeV challenge, 104 tissue culture 50% infectious doses (TCID50) of the Bilthoven strain of MeV were instilled intratracheally into anesthetized animals 17 months after vaccination. Six MeV-seronegative macaques (15U, 46U, 55U, 67U, 40V, and 43V) challenged with the same virus inoculum within a year after the immunized macaques were used for comparison. Macaques were shaved and then monitored for development of a rash every 3 to 4 days after challenge. Heparinized blood was collected to assess viremia and immune responses. Sterile cotton swabs prewetted in phosphate-buffered saline (PBS) were used to collect secretions and cells from both nares to assess viral shedding.
Cells, viruses, and virus assays.
Vero and Vero/hSLAM (49) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The Chicago-1 strain of MeV was grown and assayed by plaque formation in Vero cells. The wild-type Bilthoven strain of MeV (a gift from A. Osterhaus, Erasmus University, Rotterdam, The Netherlands) was grown in phytohemagglutinin-stimulated human cord blood mononuclear cells and assayed by syncytium formation in B95-8 cells. Viremia was assessed by cocultivation of PBMCs with B95-8 cells in RPMI supplemented with 10% FBS, penicillin, and streptomycin or Vero/hSLAM cells in DMEM supplemented with 10% FBS and streptomycin. Cocultures were scored for syncytia at 96 h, and data are reported as number of syncytia/106 PBMCs.
MeV RNA was measured by quantitative reverse transcription-PCR (RT-PCR) as previously described (8). Briefly, RNA was isolated, and the N gene was amplified from 2 × 106 PBMCs (Applied Biosystems Prism 7700) using TaqMan primers and probe. Copy number was determined by construction of a standard curve from 1 to 106 copies of RNA synthesized by in vitro transcription from a plasmid carrying the Edmonston N gene. The sensitivity of the assay was 10 copies. Data were normalized to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control and expressed as (copies of MeV N RNA/copies of GAPDH RNA) × 5,000. For nasal swab samples, products from RT-PCR using MeV N-specific primers on RNA extracted from cell pellets were run on gels and read as positive or negative.
Antibody assays.
The plaque reduction neutralization test (PRNT) was used to measure neutralizing antibody to MeV. In brief, the Chicago-1 strain of MeV was mixed with serially diluted plasma and assayed for plaque formation on Vero cells. The dilution of plasma that resulted in 50% plaque reduction was calculated.
Enzyme immunoassays (EIAs) were used to measure IgG and IgM. Maxisorp plates (Nunc) were coated with MeV-infected Vero cell lysate (Advanced Biotechnologies; 1.16 µg protein/well), lysates of L-cells expressing MeV H or F (50), or baculovirus-expressed MeV N (51). Plates were incubated with serially diluted plasma in duplicate overnight at 4°C. Secondary antibodies were horseradish peroxidase-conjugated goat antibodies to monkey IgG or IgM (Nordic) and were developed with TMB (3,3′,5,5′-tetramethylbenzidine) substrate. To assess avidity of MeV-specific antibody, increasing concentrations (0.5 to 3 M) of ammonium thiocyanate (NH4SCN), a chaotropic agent, were added to the EIA reaction mixture for 15 min. The avidity index was calculated as the concentration of NH4SCN at which 50% of the bound antibody was eluted.
T-cell assays.
ELISpot assays were used to measure MeV-specific IFN-γ-producing cells. ELISpot plates were coated with antibody to human IFN-γ (BD Bioscience), and 5 × 105 fresh PBMCs were added in the presence of 1 µg/ml pooled H, F, or N peptides, 5 µg/ml concanavalin A, or medium alone. After 40 h of incubation, plates were washed and incubated with biotinylated antibody to IFN-γ (Mabtech) followed by horseradish peroxidase (HRP)-conjugated avidin (Vector). Assays were developed with stable diaminobenzidine (DAB) solution, read on an ImmunoSpot plate reader (Cellular Technology), and analyzed using ImmunoSpot version 3.0 software.
Flow cytometry with intracellular cytokine staining was used to assess the functionality of MeV-specific T cells. Experimental procedures and gating strategies have been described previously (32). In brief, 106 fresh PBMCs were stimulated with staphylococcal enterotoxin B (SEB) (Sigma-Aldrich), medium alone, or pooled MeV H or N peptides in the presence of anti-human CD107a (BD), brefeldin A (Sigma-Aldrich), and Golgistop (BD Biosciences) for 12 h. Cells were then stained with ViViD live-dead discriminator (Invitrogen) and antibodies to CD3, CD4, CD8, CD14 (BD Bioscience), and CD20 (eBioscience). Cells were permeabilized using the BD Cytofix/Cytoperm kit and stained for intracellular IFN-γ, IL-2 (BD Bioscience), and TNF (eBioscience). Cells were read on a BD LSR II or FACSCanto II flow cytometer. A total of 400,000 events were collected per sample. Analysis was performed using FlowJo (version 8.8.7; TreeStar, Inc.) and SPICE (version 4.3.1; Mario Roederer and Joshua Nozzi, NIAID, NIH) software. After elimination of artifacts from acquisition noise, doublets, and dead cells, cytokine-producing cells were gated from CD3+ CD4+ and CD3+ CD8+ cells.
SUPPLEMENTAL MATERIAL
A no. 646 nebulizer with a Pulmo Aid compressor (DeVilbiss Health Care) and a standard mouthpiece were used to deliver a single-dose of the live attenuated Edmonston-Zagreb measles vaccine to macaques in the nebulizer-immunized group (1T, 18T, and 22T). Download
Flow cytometry analysis of T-cell function after wild-type MeV challenge. MeV-specific T cells were analyzed by intracellular cytokine staining and multicolor flow cytometry. A total of 106 fresh PBMCs were stimulated with medium alone, pooled H or N peptides (20-mers overlapping by 11 amino acids [1 µg/ml]) in the presence of anti-CD107a antibody, brefeldin A, and Golgistop for 12 h. After elimination of doublets and dead cells, cells expressing IFN-γ, TNF, IL-2, or CD107 were gated from CD3+ CD4+ cells and CD3+ CD8+ cells. Download
ACKNOWLEDGMENTS
These studies were supported by research grants from the Bill and Melinda Gates Foundation and Becton Dickinson (D.E.G.) and by Marjorie Gilbert (W.-H.W.L.) and Gilbert Otto (C.-H.P.) fellowship awards.
We appreciate the helpful advice of John Mikszta, Vince Sullivan, and Brandi Ford from BD Technologies.
Footnotes
Citation Lin WW, Pan C, Adams RJ, Laube BL, Griffin DE. 2014. Vaccine-induced measles virus-specific T cells do not prevent infection or disease but facilitate subsequent clearance of viral RNA. mBio 5(2):e01047-14. doi:10.1128/mBio.01047-14.
REFERENCES
- 1. Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. 2013. Accelerating next-generation vaccine development for global disease prevention. Science 340:1232910. 10.1126/science.1232910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plotkin SA. 2013. Complex correlates of protection after vaccination. Clin. Infect. Dis. 56:1458–1465. 10.1093/cid/cit048 [DOI] [PubMed] [Google Scholar]
- 3. Robinson HL, Amara RR. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25–S32. 10.1038/nm1212 [DOI] [PubMed] [Google Scholar]
- 4. Swadling L, Klenerman P, Barnes E. 2013. Ever closer to a prophylactic vaccine for HCV. Expert Opin. Biol. Ther. 13:1109–1124. 10.1517/14712598.2013.791277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiffner T, Sattentau QJ, Dorrell L. 2013. Development of prophylactic vaccines against HIV-1. Retrovirology 10:72. 10.1186/1742-4690-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin WH, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. 2012. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc. Natl. Acad. Sci. U. S. A. 109:14989–14994. 10.1073/pnas.1211138109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch RL, Griffin DE, Johnson RT, Cooper SJ, Lindo de Soriano I, Roedenbeck S, Vaisberg A. 1984. Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin. Immunol. Immunopathol. 31:1–12. 10.1016/0090-1229(84)90184-3 [DOI] [PubMed] [Google Scholar]
- 10. Tamashiro VG, Perez HH, Griffin DE. 1987. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr. Infect. Dis. J. 6:451–454. 10.1097/00006454-198705000-00007 [DOI] [PubMed] [Google Scholar]
- 11. Von Pirquet C. 1908. Verhalten der kutanen tuberkulin-reaktion wahrend der Masern. Deutsch. Med. Wochenschr. 34:1297–1300. 10.1055/s-0028-1135624 [DOI] [Google Scholar]
- 12. Panum P. 1938. Observations made during the epidemic of measles on the Faroe Islands in the year 1846. Med. Classics 3:829–886 [Google Scholar]
- 13. Lievano F, Galea SA, Thornton M, Wiedmann RT, Manoff SB, Tran TN, Amin MA, Seminack MM, Vagie KA, Dana A, Plotkin SA. 2012. Measles, mumps, and rubella virus vaccine (M-M-RII): a review of 32 years of clinical and postmarketing experience. Vaccine 30:6918–6926. 10.1016/j.vaccine.2012.08.057 [DOI] [PubMed] [Google Scholar]
- 14. Fontana JM, Bankamp B, Bellini WJ, Rota PA. 2008. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology 374:71–81. 10.1016/j.virol.2007.12.031 [DOI] [PubMed] [Google Scholar]
- 15. Graves M, Griffin DE, Johnson RT, Hirsch RL, de Soriano IL, Roedenbeck S, Vaisberg A. 1984. Development of antibody to measles virus polypeptides during complicated and uncomplicated measles virus infections. J. Virol. 49:409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ota MO, Ndhlovu Z, Oh S, Piyasirisilp S, Berzofsky JA, Moss WJ, Griffin DE. 2007. Hemagglutinin protein is a primary target of the measles virus-specific HLA-A2-restricted CD8+ T cell response during measles and after vaccination. J. Infect. Dis. 195:1799–1807. 10.1086/518006 [DOI] [PubMed] [Google Scholar]
- 17. van Els CA, Nanan R. 2002. T cell responses in acute measles. Viral Immunol. 15:435–450. 10.1089/088282402760312322 [DOI] [PubMed] [Google Scholar]
- 18. Jaye A, Herberts CA, Jallow S, Atabani S, Klein MR, Hoogerhout P, Kidd M, van Els CA, Whittle HC. 2003. Vigorous but short-term gamma interferon T-cell responses against a dominant HLA-A*02-restricted measles virus epitope in patients with measles. J. Virol. 77:5014–5016. 10.1128/JVI.77.8.5014-5016.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Swart RL, Yüksel S, Langerijs CN, Muller CP, Osterhaus AD. 2009. Depletion of measles virus glycoprotein-specific antibodies from human sera reveals genotype-specific neutralizing antibodies. J. Gen. Virol. 90:2982–2989. 10.1099/vir.0.014944-0 [DOI] [PubMed] [Google Scholar]
- 20. Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036–1042. 10.1093/infdis/162.5.1036 [DOI] [PubMed] [Google Scholar]
- 21. Ruckdeschel JC, Graziano KD, Mardiney MR., Jr. 1975. Additional evidence that the cell-associated immune system is the primary host defense against measles (rubeola). Cell. Immunol. 17:11–18. 10.1016/S0008-8749(75)80002-5 [DOI] [PubMed] [Google Scholar]
- 22. Guidotti LG, Chisari FV. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65–91. 10.1146/annurev.immunol.19.1.65 [DOI] [PubMed] [Google Scholar]
- 23. Binder GK, Griffin DE. 2001. Interferon-γ−mediated site specific clearance of alphavirus from CNS neurons. Science 293:303–306. 10.1126/science.1059742 [DOI] [PubMed] [Google Scholar]
- 24. Topham DJ, Tripp RA, Doherty PC. 1997. CD8+ T cells clear influenza virus by perforin or fas-dependent processes. J. Immunol. 159:5197–5200 [PubMed] [Google Scholar]
- 25. Walker BD, Burton DR. 2008. Toward an AIDS vaccine. Science 320:760–764. 10.1126/science.1152622 [DOI] [PubMed] [Google Scholar]
- 26. Simmonds P. 2004. Genetic diversity and evolution of hepatitis C virus—15 years on. J. Gen. Virol. 85:3173–3188. 10.1099/vir.0.80401-0 [DOI] [PubMed] [Google Scholar]
- 27. Ndung'u T, Weiss RA. 2012. On HIV diversity. AIDS 26:1255–1260. 10.1097/QAD.0b013e32835461b5 [DOI] [PubMed] [Google Scholar]
- 28. Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. 10.1126/science.1241144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albrecht P, Ennis FA, Saltzman EJ, Krugman S. 1977. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91:715–718. 10.1016/S0022-3476(77)81021-4 [DOI] [PubMed] [Google Scholar]
- 30. Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527–532. 10.1001/jama.280.6.527 [DOI] [PubMed] [Google Scholar]
- 31. Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J. Infect. Dis. 196:1339–1345. 10.1086/522519 [DOI] [PubMed] [Google Scholar]
- 32. Lin WH, Griffin DE, Rota PA, Papania M, Cape SP, Bennett D, Quinn B, Sievers RE, Shermer C, Powell K, Adams RJ, Godin S, Winston S. 2011. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 108:2987–2992. 10.1073/pnas.1017334108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bouche FB, Ertl OT, Muller CP. 2002. Neutralizing B cell response in measles. Viral Immunol. 15:451-471. 10.1089/088282402760312331 [DOI] [PubMed] [Google Scholar]
- 34. Ryon JJ, Moss WJ, Monze M, Griffin DE. 2002. Functional and phenotypic changes in circulating lymphocytes from hospitalized Zambian children with measles. Clin. Diagn. Lab. Immunol. 9:994–1003. 10.1128/CDLI.9.5.994-1003.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. 2001. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J. Infect. Dis. 183:532–538. 10.1086/318533 [DOI] [PubMed] [Google Scholar]
- 36. Riddell MA, Moss WJ, Hauer D, Monze M, Griffin DE. 2007. Slow clearance of measles virus RNA after acute infection. J. Clin. Virol. 39:312–317. 10.1016/j.jcv.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 37. Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. 2007. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J. Virol. 81:8468–8476. 10.1128/JVI.00228-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81:12071–12076. 10.1128/JVI.01261-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 40. Low N, Kraemer S, Schneider M, Restrepo AM. 2008. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine 26:383–398. 10.1016/j.vaccine.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 41. de Swart RL, LiCalsi C, Quirk AV, van Amerongen G, Nodelman V, Alcock R, Yüksel S, Ward GH, Hardy JG, Vos H, Witham CL, Grainger CI, Kuiken T, Greenspan BJ, Gard TG, Osterhaus AD. 2007. Measles vaccination of macaques by dry powder inhalation. Vaccine 25:1183–1190. 10.1016/j.vaccine.2006.10.019 [DOI] [PubMed] [Google Scholar]
- 42. Polack FP, Auwaerter PG, Lee SH, Nousari HC, Valsamakis A, Leiferman KM, Diwan A, Adams RJ, Griffin DE. 1999. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 5:629–634. 10.1038/9473 [DOI] [PubMed] [Google Scholar]
- 43. Hoffman SJ, Polack FP, Hauer DA, Singh M, Billeter MA, Adams RJ, Griffin DE. 2003. Vaccination of rhesus macaques with a recombinant measles virus expressing interleukin-12 alters humoral and cellular immune responses. J. Infect. Dis. 188:1553–1561. 10.1086/379250 [DOI] [PubMed] [Google Scholar]
- 44. Polack FP, Hoffman SJ, Moss WJ, Griffin DE. 2003. Differential effects of priming with DNA vaccines encoding the hemagglutinin and/or fusion proteins on cytokine responses after measles virus challenge. J. Infect. Dis. 187:1794–1800. 10.1086/375245 [DOI] [PubMed] [Google Scholar]
- 45. Good RA, Zak SJ. 1956. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics 18:109–149 [PubMed] [Google Scholar]
- 46. Albertyn C, van der Plas H, Hardie D, Candy S, Tomoka T, Leepan EB, Heckmann JM. 2011. Silent casualties from the measles outbreak in South Africa. S. Afr. Med. J. 101:313–317 http://www.samj.org.za/index.php/samj/article/view/4616/3217 [DOI] [PubMed] [Google Scholar]
- 47. Permar SR, Klumpp SA, Mansfield KG, Kim WK, Gorgone DA, Lifton MA, Williams KC, Schmitz JE, Reimann KA, Axthelm MK, Polack FP, Griffin DE, Letvin NL. 2003. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J. Virol. 77:4396–4400. 10.1128/JVI.77.7.4396-4400.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Permar SR, Klumpp SA, Mansfield KG, Carville AA, Gorgone DA, Lifton MA, Schmitz JE, Reimann KA, Polack FP, Griffin DE, Letvin NL. 2004. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J. Infect. Dis. 190:998–1005. 10.1086/422846 [DOI] [PubMed] [Google Scholar]
- 49. Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399–4401. 10.1128/JVI.75.9.4399-4401.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beauverger P, Buckland R, Wild F. 1993. Establishment and characterisation of murine cells constitutively expressing the fusion, nucleoprotein and matrix proteins of measles virus. J. Virol. Methods 44:199–210. 10.1016/0166-0934(93)90055-V [DOI] [PubMed] [Google Scholar]
- 51. Hummel KB, Erdman DD, Heath J, Bellini WJ. 1992. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J. Clin. Microbiol. 30:2874–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A no. 646 nebulizer with a Pulmo Aid compressor (DeVilbiss Health Care) and a standard mouthpiece were used to deliver a single-dose of the live attenuated Edmonston-Zagreb measles vaccine to macaques in the nebulizer-immunized group (1T, 18T, and 22T). Download
Flow cytometry analysis of T-cell function after wild-type MeV challenge. MeV-specific T cells were analyzed by intracellular cytokine staining and multicolor flow cytometry. A total of 106 fresh PBMCs were stimulated with medium alone, pooled H or N peptides (20-mers overlapping by 11 amino acids [1 µg/ml]) in the presence of anti-CD107a antibody, brefeldin A, and Golgistop for 12 h. After elimination of doublets and dead cells, cells expressing IFN-γ, TNF, IL-2, or CD107 were gated from CD3+ CD4+ cells and CD3+ CD8+ cells. Download